
| HOME | SITEMAP | CONTACT US | DIRECTIONS |
| Recent Publications |
| Contact Info |

Christof T. Grewer, Ph.D
Professor, Biological and Physical Chemistry
- Ph.D. in Physical Chemistry, Johann Wolfgang Goethe-University, Frankfurt, Germany, 1993
- Postdoctoral Fellow, Cornell University, Ithaca, NY, 1993-1996
- Senior Research Associate, Max-Planck-Institute for Biophysics, Frankurt, Germany, 1997-2003
- Assistant Professor, Dept. of Physiology and Biophysics, University of Miami School of Medicine, Miami, FL, 2003-2007
- Member, Neuroscience Program, University of Miami School of Medicine, Miami, FL, 2003-2007
- Associate Professor, Dept of Chemistry, Binghamton University, Binghamton, NY, 2008-2011
- Professor, Dept of Chemistry, Binghamton University, Binghamton, NY, 2011-
Research Interests
The long-term goal of our research is to understand the function and the working mechanism of membrane-bound transport proteins. In general, transporters use different types of energy sources to actively move specific substrates, such as inorganic ions or small, organic molecules across the membrane into or out of cells. Recently, significant progress has been made towards our understanding of the molecular architecture through the availability of high-resolution structures of several transporters in several different conformations. However, the structural pictures are static leaving many questions regarding the actual transport mechanism(s) unresolved. Our aim is to combine functional, structural and computational evidence in order to obtain an understanding of how these transport proteins work.
Our current research focuses mainly on secondary-active Na+-coupled transporters, which are energized by coupling of substrate transport to the cotransport of sodium ions down their electrochemical potential gradient across the membrane. Neurotransmitter transporters and amino acid transporters belong to this class of transport proteins. The systems currently investigated are glutamate transporters, which contribute to the removal of the excitatory neurotransmitter glutamate from the synapse, and neutral amino acid transporters (SNATs, ASCTs, SLC6A14), which catalyze import or export of glutamine and other important neutral amino acids into or from cells.
Dynamics of the transport process
In many cases, membrane transport is associated
with stationary or transient transport of charge. We measure this
charge transport with electrophysiological techniques, such as current
recording from transporter-expressing, voltage-clamped whole cells or
excised inside-out patches. In order to investigate transient charge
transport, we perturb a pre-existing transporter steady state by
applying voltage or rapid substrate concentration jumps and subsequently
measuring the kinetics of the relaxation to a new steady state with a
sub-millisecond time resolution. 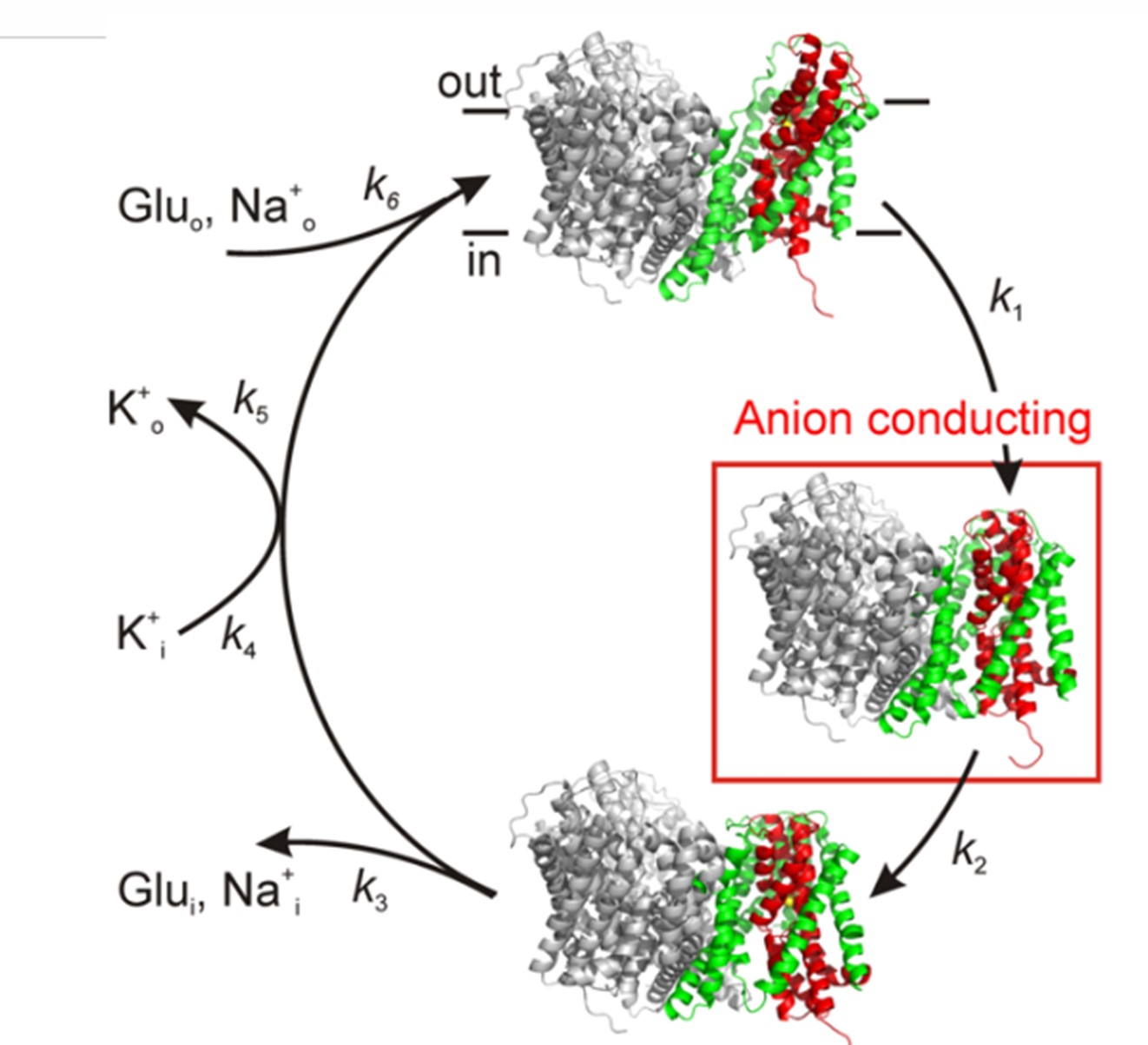 A hypothetical transport mechanism (see Figure)
combines evidence from such pre-steady-state functional data with
structural information for the glutamate
transporters. This mechanism predicts that two structural changes are
associated with transmembrane glutamate movement: 1) The closing of an
external gate after substrate binding, and 2) the subsequent opening
of an internal gate, allowing dissociation of substrate to the
cytoplasm.
A hypothetical transport mechanism (see Figure)
combines evidence from such pre-steady-state functional data with
structural information for the glutamate
transporters. This mechanism predicts that two structural changes are
associated with transmembrane glutamate movement: 1) The closing of an
external gate after substrate binding, and 2) the subsequent opening
of an internal gate, allowing dissociation of substrate to the
cytoplasm.
Development of pharmacological tools
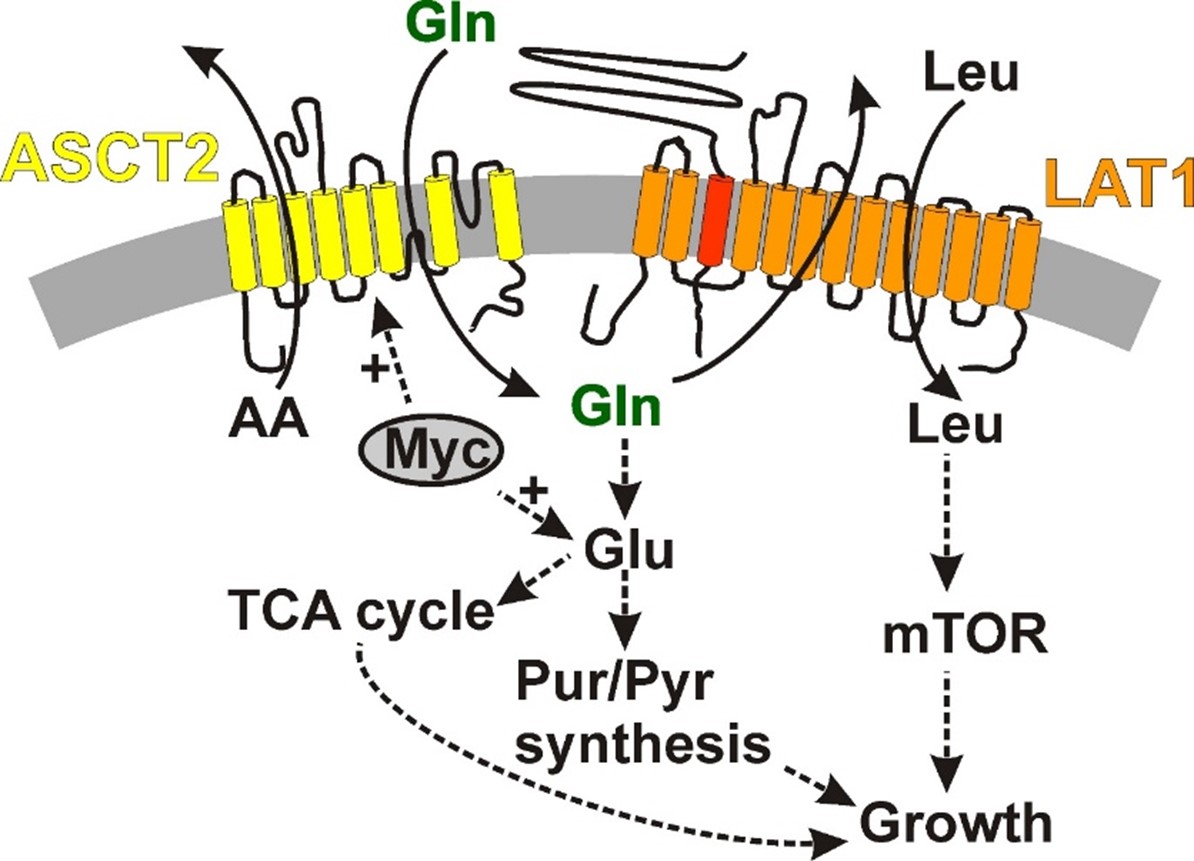
We are also interested in developing pharmacological tools for the investigation of neutral amino acid transporters. Several transporters for glutamine, in particular, are overexpressed in cancer cells, where they supply the cells with glutamine as an energy and nitrogen source. The aim of this research is to block glutamine transporters, to prevent cell growth by limiting glutamine supply. The following figure shows the interplay of neutral amino acid transporters ASCT2 and LAT1 in the biochemical pathways leading to cell growth. We have recently synthesized compounds that inhibit the neutral amino acid transporter ASCT2 with a 20μM apparent affinity. These compounds were identified using an in-silico docking approach, in collaboration with the Schlessinger laboratory at Mount Sinai School of Medicine, followed by validation of the docking results with electrophysiological analysis of inhibitor-protein interaction. We are continuing the development of those compounds with the aim to find inhibitors with nM affinities. In addition, we are identifying allosteric inhibitors for ASCT2.
Computational studies
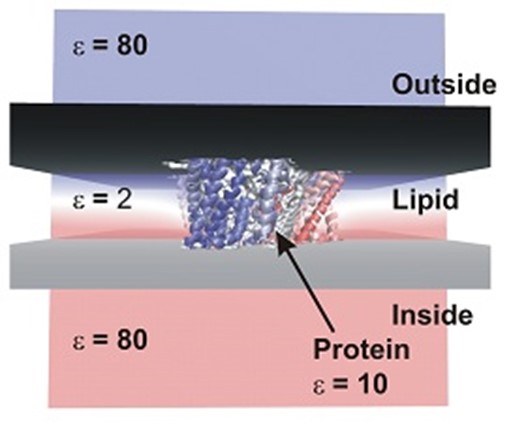
Investigation of secondary-active transport by using computational methods has traditionally focused on understanding the dynamics of the alternating access mechanism. However, there is a lack of computational analysis of electrical properties of electrogenic transporters. We are using numerical analysis of the Poisson-Boltzmann equation to compute valences and charge movement associated with partial reactions in the transport cycle. These partial reactions are substrate/ion binding/dissociation reactions, as well as structural changes associated with alternating access and gating. Structural information for the Poisson-Boltzmann analysis is obtained from available x-ray structures, or Molecular Dynamics (MD) simulations. An example of the glutamate transporter in an implicit membrane of dielectric constant of 2 is shown in the adjacent Figure. The results from these computations predict sign and magnitude of charge movement, which can be directly validated by experimental analysis through patch clamping.
