|
|
|
| |
|
|
Latest News |
|
| |
 |
| |
Author: Sarah Millar
Published Date: 18 January 2014
Source / Publisher: Chemistry
–
A European Journal/Wiley-VCH
Copyright: Wiley-VCH Verlag
GmbH & Co. KGaA, Weinheim |
| |
Hydrogenation of olefins is often used to test new heterogeneous
catalysts, especially noble metals, such as Pt, Pd, and Au.
Each crystallographic facet of such catalysts has its own role
in the catalysis. For example, {111} planes of Au preferentially
activate C=O groups, whereas low-coordinated sites at corners
and edges favor the breaking of C=C bonds. It has also been
shown that hydrogenation of styrene takes place on defect sites
(high-index kinks and steps) of Pd catalysts, rather than their
low-index terraces. Thus, the fabrication of nanocatalysts with
high-index crystallographic facets could lead to more efficient
catalysts. |
| |
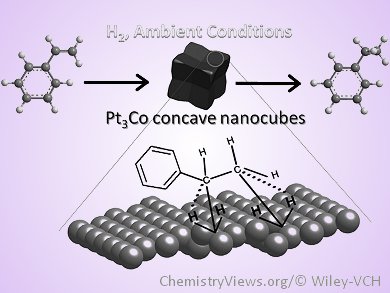
We recently report the simple synthesis of high-quality Pt3Co
nanocubes (NCs) with a concave structure. The NCs were prepared
through a wet-chemical approach, in which the ratio of oleyl
amine (OAM)/oleic acid (OA) was finely tuned. The NCs are
terminated with high-index crystallographic planes containing a
combination of several sub-facets.The team proposes that the
formation of Pt3Co seeds is dependent on the concentration of
free metallic atoms during the reaction, whereas the final
morphology of NCs was controlled by selective binding with the
appropriate OAM/OA ratio during the crystal-growth stage.Using
hydrogenation of styrene as a model reaction, the NCs showed
enhanced catalytic activity in comparison with low-indexed
surface terminated Pt3Co nanocubes of similar size
owing to their more open structure, more stable
composition/morphology, and the increased number of active
atomic sites located on their high-index crystallographic
planes. |
| |
The
manuscript (DOI: 10.1002/chem.201301724) has been chosen to be
highlighted on the
ChemistryViews website. The short news article is available
at this
link. |
| |
|
|
[10/17/2012] |
Watching Nanoscale Octahedra Crystallize |
| |
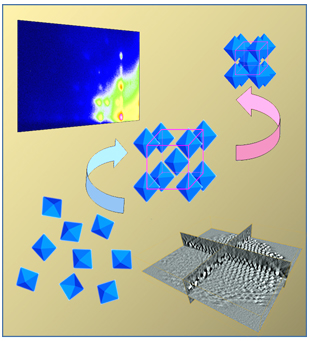
Assembly of nanoparticles into ordered
superlattices opens up many potential applications such as
sensors, catalysts, and novel optical materials. Unlike
spherical particles, which can normally self-assemble into a
close-packed fcc superlattice, systems containing
non-spherical building blocks are much less known. We have
recently demonstrated how to synthesize monodisperse
non-spherical nanocrystals, including nanocubes and nanoctahedra.
In recent experiments at D1 station of Cornell High Energy
Synchrotron Source (CHESS) at Cornell University, together with
CHESS staff scientist Dr. Detlef-M. Smilgies we determined an
extremely low packing density superstructure consisting of Pt3Cu2
nanotahdera. Furthermore, for the first time, we observed reversible
Kirkwood-Alder transition
occurred in this system. The details were just highlighted on
CHESS web site
http://news.chess.cornell.edu/articles/2012/Fang10172012.html
as
well as
reported in our recent publication [JACS
134 (34), 14043-14049 (2012)].
|
| |
 |
| |
High-Pressure Studies Uncover Ways to Make Valuable
Technological Materials [12/20/2011] |
| |
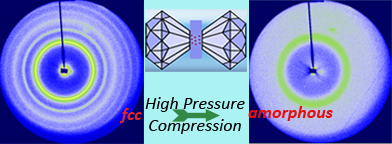
New materials with surprising and sometimes useful new
properties are often found by modifying the structures of known
materials without altering their composition. One approach is
to subject materials to very high pressures of up to 100 GPa
(100GPa = 1,000,000 times atmosphere pressure). The classic
example is graphite, a soft and opaque material with a loose
layered structure, which can be turned into ultra-hard,
transparent diamond, a crystal with cubic symmetry.
Unlike diamond, which retains its structure even after the
pressure is released, most high pressure structures are
metastable, and return to their unpressurized form when pressure
is released. Thus, for example, sulfur, which is
superconducting under pressure, is not useful as a
superconductor because this property is lost when the pressure
is removed.
A
long term goal of many materials scientists is to make high
pressure materials that retain their properties even at ambient
pressure. Recently, researchers
from the chemistry and materials
science departments of the State University of New York at
Binghamton and Cornell University
have joined forces to make progress toward this goal. They
demonstrated for the first time that the semiconductor PbTe has
a high-pressure-tuned metastable structure that can be retained
at ambient conditions. This material also shows, for the first
time, a reversal of a so-called Hall-Petch relation relating the
structural stability to particle size. In addition, they went a
step further to show that by improving the protocol for
synthesis, useful forms of PbTe can be created without any
pressure at all. This important result raises the possibility
that PbTe semiconductor materials could someday serve a host of
useful technological applications, such as thermo-electronics,
energy conversion, etc. Details of such study was released in
our recent publication:
Nano Lett., 2011, 11(12),
5531-5536.
 |
| |
|
| [06/09/2011] |
Our
previous publication has been listed as one of the top 20 most
cited articles
that
were published
in
Nano Letter
within
the last 3 years. |
| |
http://pubs.acs.org/action/showMostCitedArticles?topArticlesType=recent&journalCode=nalefd |
| |
|
| [01/18/2011] |
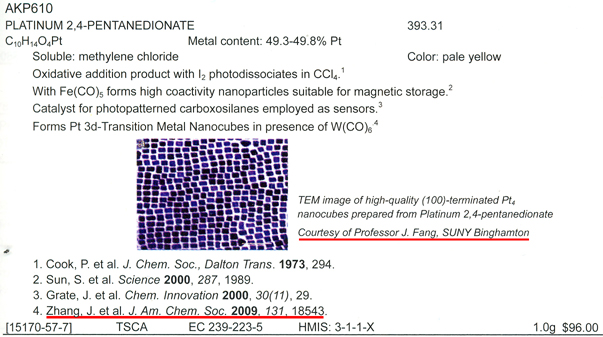
Our previous
synthesis methodology of
Pt nanocubes was
cited in
gelest
catalog, "Metal-Organics"
(p.
176-177; follow the
link, and click "show details".) |
| |
 |
| [11/22/2010] |
|
|
Are shape-controlled nanocrystals
of Pt-based alloy promising electrocatalysts? |
| Our previous work,
Pt3M (M=Fe, Co, Ni and Pt)
nanocube synthesis and
ORR study on Pt3Ni nanoctahedra,
has been highlighted by Xia's paper, "
|
| |
|
| [08/12/2010] |
Can an electrocatalytic
investigation be conducted on a particle shape-controlled level? |
| We just
receive word that our communication "Enhancing by Weakening: |
| |
Electrooxidation of Methanol on Pt3Co and Pt Nanocub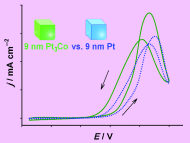 es"
has been chosen as a "Hot Paper" by the Editors of Angewandte Chemie
for its importance in a rapidly evolving field of high current
interest. This announcement will be published in the table of
contents entry on Angewandte's homepage prior to publication of the
full paper as soon as possible. es"
has been chosen as a "Hot Paper" by the Editors of Angewandte Chemie
for its importance in a rapidly evolving field of high current
interest. This announcement will be published in the table of
contents entry on Angewandte's homepage prior to publication of the
full paper as soon as possible.
Read more...
|
| [04/27/2010] |
It is
now possible to assemble binary nanosphere superlattice patterns
|
| |
with various structures, and
it was determined that opposite electrical charges are the major
driving force to result in such patterns. With a success of various nanopolyhedron syntheses, the question has been extended to a
non-spherical nanoparticle colloidal system: What is the dominant driving
force to induce a superlattice assembly in a
non-spherical-particle system?
With our recent advances in
preparation of shape- and size-controlled nonspherical nanoparticles,
we answered this question in the current issue of ACS Nano
(4/4). In this work, we reported our observation of two-dimensional
(2D) superlattices consisting of c-In2O3
nanoctahedra and Pd nanospheres, and identified three types of c-In2O3
skeleton structures in the 2D octahedral c-In2O3-spherical
Pd nanoparticle supercrystal patterns. In collaboration with Dr. Luo at TAMU, we concluded that (1) the
vertices of c-In2O3 nanoctahedra could have
higher electrical charge density than that on edge or plane; (2)
most of the Pd nanoparticles locate on the middle plane of the c-In2O3
nanoctahedra well above the substrate surface (support film) rather
than sitting on it in the 2D supercrystal assembly patterns; (3) the
orientation of c-In2O3 nanoctahedra dominates
the structure of a nonspherical 2D supercrystal pattern. On the
basis of these investigations in a non-spherical nanoparticle
colloidal system, we confirmed that Coulomb forces resulted from opposite electrical charges on nanopolyhedra (c-In2O3)
and metal nanospheres (Pd) are the major driving forces to induce
such assemblies. |
| |
Read more... |
| [01/06/2010] |
Research
Highlight: Shape DOES
affect the electrochemical catalytic activity. |
| |
We
successfully synthesized high-quality Pt3Ni nanopolyhedra
(geometric tiny
solids with flat facets and straight edges)
using a recently developed wet-chemical approach,
and investigated their
shape-dependent oxygen reduction activity which is a significant
reaction in the cathode of
proton exchange membrane
fuel cells. Amazingly, we
determined that the activity on Pt3Ni nanoctahedra
(terminated with {111} facets) is ~5 folds higher than that of
nanocubes (bounded
with {100} facets) with a similar size. This discovery is important
for developing new type of electrocatalyst with a superior oxygen
reduction activity used in the real world of fuel cells. This result
will be published in
Nano Letters
soon. |
| |
|
| [11/25/2009] |
We
released a facile and general synthetic method for preparation of
high- |
| |
quality,
{100}-terminated Pt3M nanocubes (M = Pt or 3d-transition
metals Co, Fe, and Ni). We realize that addition of W(CO)6
is crucial for control of the nucleation process when the metallic
precursors are reduced, whereas an optimized ratio of the solvent
pair, oleylamine and oleic acid, is the key to enabling the lowest
total surface energy on {100} facets in order to develop such cubic
nanocrystals in the present system. This novel method was published
in the last issue of
JACS
this year. |
| |
 |
| |
|
 |
|
[02/04/2009]
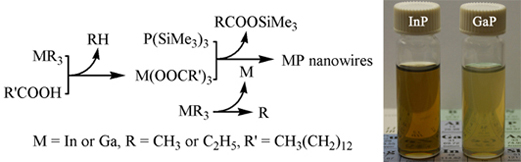
Zhaoping's article, "Soluble
InP and GaP Nanowires: Self-Seeded, Solution-Liquid-Solid
Synthesis and Electrical Properties" was accepted by Chem. Eur. J. In recent years, a solution-based
approach supported by a solution-liquid-solid (SLS) mechanism
has been developed to enable the
quality-control in addition to previous physical
processing methods.
Nevertheless, there still has a large room to improve the
wet-chemical synthesis of III-V nanowires. Our modified
strategy
presented in this
publication
avoids some issues that exist in
the
SLS method, such as heterogeneous phase of catalyst and potential
metallic contamination in nanowires.
We also demonstrate excellent quality of the as-prepared InP and
GaP nanowires, including low native point defects for carrier
concentrations and few structural defects (especially for InP).
The scientific impacts of this work lie in the fact that in this
relatively facile solution-based approach developed in our group
the catalyst seeds can be in-situ generated by the decomposition of the metalorganic precursor,
In(CH3)3 or Ga(C2H5)3,
at an appropriately high temperature without using any special
surfactant. Moreover, this synthetic approach
may be promising in readily extending to a
preparation strategy of other high-quality III-V nanowires
including GaAs, GaSb, InAs, InSb, and their (Ga/In)(P/As/Sb)
alloys.
Read more... |
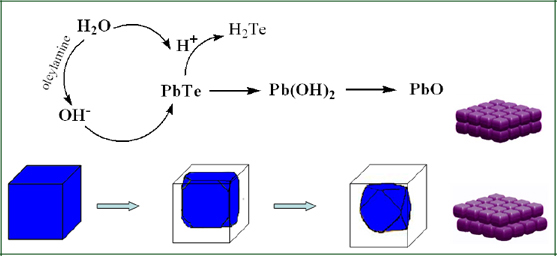
| [09/22/2008]
Jun's research
article, "Simple
Cubic Super Crystals Containing PbTe
Nanocubes
and Their Core-Shell Building Blocks",
was published in JACS. In this full paper,
We
report |
 |
|
a
preparation of high-quality cubic
PbTe nanocrystals, their square-array assemblies in
two-dimensional patterns, as well as simple cubic super
crystals. The influence of oleylamine in nanocrystal synthesis
and core-shell formation through an anion-exchange mechanism was
also studied. The simple cubic super crystals together with
two-dimensional assembly patterns containing PbTe nanocubes and
their core-shell building blocks were fully characterized. Such
super crystals consisting of structural building blocks may
allow engineering of more complex materials from which novel
properties may emerge.
Read more... |
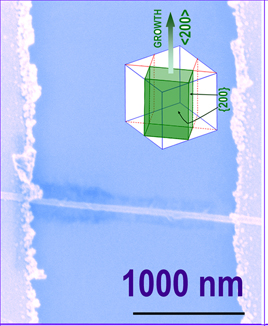 |
[09/09/2008]
Jun's research communication, entitled "p-Type
Field-Effect Transistors of Single-Crystal ZnTe Nanobelts",
was
published in Angewandte
Chemie Int. Ed.
In
this work, we
have been succeeded, for the first time, in preparation of
straight ZnTe nanobelts with extremely low thickness (< 6 nm).
We further determined a crystal growth direction of <
(-2)0 0 >
in the growth of the belts,
which has never been reported previously.
We also
demonstrated these single-crystal ZnTe nanobelts as p-type
field-effect transistors, revealing a bright future of
applications in nanodevice engineering.
Read more... |
| [03/19/2008]
Our self-assembly paper, entitled "Super-Crystal Structures of
Octahedral c-In2O3
Nanocrystals", was published in JACS. Three-dimensional (3D)
self-assembly of nanocrystal (NC) superlattice, i.e. super crystal (SC), has
attracted increasing attention. The small building blocks for assemblies
are usually spherical nanocrystals. Recent progress indicates that it is
possible to achieve a super crystal using non-spherical NCs, such as cubic
NCs. |
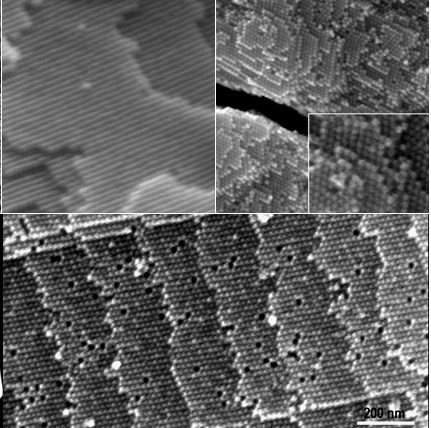 |
| In this article,
we
describe and analyze 2D and some 3D assemblies of uniform cubic-phase In2O3
NCs with octohedral shapes. We demonstrate our amazing
observations on this kind of super crystals (or superlattices) as a
model system, exposing largeness at least in tens of microns scale and
other unique features such as steps, terraces, kinks and vacancies which
are similar to those from a single crystal. Based on the EM
observations, three types of well-defined octahedral NC
packing structures in such super crystal system are also identified.
Read more...
|
 |
[2/13/2008]
Zhaoping's
III-V QD paper was published in Angewandte Chemie and highlighted as
VIP
(Very Important Paper) on Feb. 13, 2007.
[Dept.
Link]
III-V semiconductors are very important materials and are of
great interest for new generation of microelectronics. Colloidal
III-V semiconductor nanocrystals have also been a subject of
intensive studies because of their rich phenomena associated
with quantum-confinement effects. However, studies of III-V
nanocrystals are largely restrained due to the difficulty of
their synthetic chemistry. The scientific impacts of this work
lie on that we have |
|
introduced a new concept, co-reduction, to understand the
formation of InP.
We believe that this report
may provide the following
breakthroughs:
(1) We demonstrate an great
improvement of the synthetic
conditions using
a
novel approach, e,g, shortening the period of reaction time and
reducing the cost of precursor; (2)
This novel method has skipped expensive or more hazardous
P-sources, such as tris(trimethylsilyl)phosphine in the
process of InP nanocrystal
synthesis,
making this synthetic strategy noticeably “greener” and more
economical.
(3) The present work may benefit the further scale-up of such
III-V chemical synthesis as well, creating
a new direction in extending this achievement to the
preparations of InAs and InSb nanocrystals using AsCl3
and SbCl3 as the pnicogen sources, respectively.
Read more... |
Last
Modified: 01/21/2014
|
|

