Professor James A. Dix
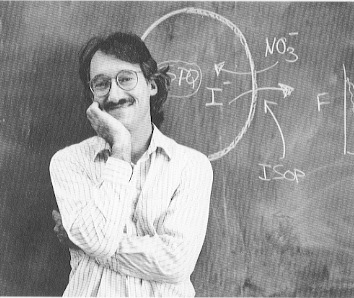
- B.A., Chemistry, Grinnell College, 1971
- Ph.D., Physical Chemistry, UCLA, 1977
- NIH Postdoctoral Fellow, Harvard Medical School, 1977-79
- Research Fellow, Cardiovascular Reserach Institute, University of California, San Francisco, 1988-89
- Visiting Scientist, Theoretical Biology and Biophysics, Los Alamos National Laboratory, 1995-1996

My research goal is to obtain a molecular description of the dynamical aspects of biophysical systems. My research has focused on membrane permeability, with particular emphasis on membranes of human red blood cells and of cells grown in culture. The physico-chemical methods we use include electron spin resonance, nuclear magnetic resonance and fluorescence spectroscopy, fluorescence microscopy and image analysis, stopped-flow and temperature-jump methods, and computer simulation. More recently, I have also become interested in exploring how the Internet can be used to teach and learn chemistry.
Back to Research Interests
Membranes are important both biologically and industrially, because they provide a very useful way of controlling the transport of chemicals between two compartments. Familiar examples of the use of industrial membranes are to desalinate and purify polluted water, and of biological membranes to control metabolite entry and waste exit from cells.
One general thrust of our research is to measure the rate at which fluorescent molecules rotate and diffuse; the rotational and diffusional rates can be used as an indication of the viscosity of environment surrounding the fluorophores. We have placed fluorophores into the cytoplasm of biological cells, and, by measuring the anisotropy of emitted fluorescence, have been able to estimate the cytoplasmic viscosity by measuring the rotational and diffusional rates of the fluorophores ["Mapping of Fluorescence Anisotropy in Living Cells by Ratio Imaging: Application to Cytoplasmic Viscocity," James A. Dix and A.S. Verkman, Biophys. J. 57, 231-240 (1990)]. These experiments were done with a fluorescence microscope and image analysis, enabling the fluorescence parameters to be resolved spatially. The spatial resolution enabled us to estimate how viscosity varies across a cell. We plan to use these methods to resolve spatially any viscosity changes occurring in biological cells in response to changes in the extracellular environment.
We have had a long interest in how electrolytes and non-electrolytes cross the human red blood cell membrane. Most recently, we have used the fluorescent probe, SPQ (6-methoxy-N-(3-sulfopropyl)quinolinuim) to study red cell chloride-bicarbonate exchange [Teresa M. Calafut and James A. Dix, "Chloride-Bicarbonate Exchange Through Human Red Cell Ghost Membranes Characterized by a Fluorescent Probe," Anal. Biochem. 230, 1-7 (1995)]
Back to Research Interests
Another recent thrust of our research is to figure out how a biological cell responds to messages sent out by regulatory parts of an organism. A primary messenger binds tightly and specifically to a receptor on the outside of the cell membrane. The binding elicits a secondary response inside the cell, with various second messengers producing the desired cellular response. We have been sorting out the mechanism by which A6 cells (a cultured cell system used as a model for membrane sodium channels) respond to treatment with isoproterenol. The experiments involve introducing fluorescent indicators of ion concentrations into the cell cytoplasm, and treating cells with various inhibitors of intracellular enzymes thought to be involved in the secondary response.
Back to Research Interests
A third thrust of our research is to determine on a molecular level how cells respond to deprivation of oxygen or nutrients, or to exposure to toxins. We have identified the mitochondria of cells as an early and sensitive indicator of cellular dysfunction. ["Rhodamine 123 as a Probe of In-Vitro Toxicity in MDCK Cells," Robert M. Lachowiez, Barbara Clayton, Kim Thallman, James A. Dix, and Robert G. VanBuskirk, Cytotechnology 2, 203-211 (1989)]. Our goal is to determine to what extent viable mitochondria can be used to define whether a cell is reversibly or irreversibly damaged.
Back to Research Interests
A more recent research interest has been to determine to what extent electronic methods can be used to teach and learn undergraduate chemistry. We have focussed initially on developing an electronic curriculum for introductory chemistry using the World Wide Web [James A. Dix, Robert A. Allendoerfer, Wayne E. Jones, Jr., Roy A. Lacey and Bernard J. Laurenzi, "An Electronic Curriculum for Introductory Chemistry", J. Instructional Technology 24, 151-157 (1995)]. In collaboration with Wayne Jones at Binghamton and Bob Allendoerfer at SUNY Buffalo, we have developed a curriculum prototype [Wayne E. Jones, Jr., James A. Dix and Robert D. Allendoerfer, "Teaching chemistry on the World Wide Web: An interactive internet learning environment for introductory chemistry", ACS symposium, New Orleans, March 24-25, 1996] that will be used at SUNY Binghamton and SUNY Buffalo in the Fall semester, 1996.
Back to Research Interests
Selected Publications
Mazin Magzoub, Hua Zhang, James A. Dix, and A. S. Verkman, “Extracellular Space Volume Measured by Two-Color Pulsed Dye Infusion with Microfiberoptic Fluorescence Photodetection,” Biophys. J. 96, 2382–2390 (2009)
James A. Dix and A.S. Verkman, “Crowding effects in solutions and cells”, Annu. Rev. Biophys. 37, 247-263 (2008)
Mazin Magzoub, Prashant Padmawar, James A. Dix, and A. S. Verkman, “Millisecond Association Kinetics of K+ with Triazacryptand-Based K+ Indicators Measured by Fluorescence Correlation Spectroscopy,” J. Phys. Chem. B 42, 21216-21221 (2006)
James A. Dix and Barb Hillery, “Living with chemicals: RAGE in the community,” Annals NY Acad. Sci. 1076, 930 (2006)
James A. Dix, Erik F.Y. Hom, and A.S. Verkman, “Fluorescence Correlation Spectroscopy Simulations of Photophysical Phenomena and Molecular Interactions: A Molecular Dynamics/Monte Carlo Approach,” J. Phys. Chem. B, 110, 1896-1906 (2006)
Dick A.F.D. Mahlangu and James A. Dix, “Halide fluxes in epithelial cells measured with n automated cell plate reader,” Analytical Biochemistry, 325, 28-34 (2004)

 Return to the faculty
Return to the faculty
 Return to the Chemistry Table of Contents
Return to the Chemistry Table of Contents


 Return to the faculty
Return to the faculty
 Return to the Chemistry Table of Contents
Return to the Chemistry Table of Contents