

A fluorescent derivative of taxol, 2-debenzoyl-2-(m-aminobenzoyl)taxol
(2-AB-taxol), has been prepared. 2-AB-taxol induces microtubule assembly
in vitro, but is less potent than taxol itself. The critical concentrations
for tubulin assembly in 10 mM phosphate buffer (pH 7.0) containing 16 mM MgCl2
and 0.1 mM GTP are 0.3 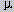 M for taxol and
1.5
M for taxol and
1.5 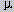 M for 2-AB-taxol
(20
M for 2-AB-taxol
(20 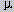 M drug).
The fluorescence emission spectrum of 2-AB-taxol in solvent undergoes a shift
to higher energy and an increase in quantum yield with decreasing solvent
polarity, consistent with a general solvent effect. Upon binding to
microtubules, the quantum yield of 2-AB-taxol increases and the emission
maximum is shifted to higher energy. In addition, tubulin fluorescence is
quenched and energy transfer from tubulin to 2-AB-taxol is apparent. These
results indicate that substituents on the C-2 position of taxol associate with
tubulin when bound to the microtubule.
M drug).
The fluorescence emission spectrum of 2-AB-taxol in solvent undergoes a shift
to higher energy and an increase in quantum yield with decreasing solvent
polarity, consistent with a general solvent effect. Upon binding to
microtubules, the quantum yield of 2-AB-taxol increases and the emission
maximum is shifted to higher energy. In addition, tubulin fluorescence is
quenched and energy transfer from tubulin to 2-AB-taxol is apparent. These
results indicate that substituents on the C-2 position of taxol associate with
tubulin when bound to the microtubule.

Taxol, a diterpene originally isolated from the bark of the Western Yew, Taxus brevifolia, has shown great utility in the treatment of certain human malignancies.[1] Taxol exerts its biological effects by interfering with microtubule dynamics in vivo. Most antimicrotubule agents, such as colchicine, podophyllotoxin, combretastatin, the Vinca alkaloids and related agents, interfere with normal microtubule processes by promoting a net depolymerization of cellular microtubules.[2] Taxol, however, appears to possess a unique mechanism of action for antimicrotubule drugs: taxol stabilizes microtubules against depolymerization, apparently through binding to a site on the microtubule rather than to soluble tubulin.[3]
The molecular mechanism(s) by which taxol binds to and stabilizes microtubules is currently unclear. There are several characteristics of the taxol-microtubule interaction that complicate the usual methods available for probing the ligand-receptor association. First, taxol possesses no natural chromophore outside of the chromophoric regions of the protein. Changes that may occur in taxol electronic spectra upon microtubule binding are obscured by the electronic spectra of the protein. Second, taxol binds preferentially to microtubules rather than tubulin.[4] The size of the receptor renders methods such as solution state NMR spectroscopy ineffectual. And third, the structural complexity of the molecule hampers synthetic efforts to create a broad spectrum of analogs for structure-activity studies. Structure-activity studies assist in defining the region(s) of the natural product necessary for high affinity binding to a receptor. This experimental approach may also guide the design of modified taxols for mechanistic studies.
One approach to investigating the mechanism of taxol binding to microtubules is to prepare a taxol analog in which a fluorescent probe is covalently attached to the molecule. For our first fluorescent probe, we chose to replace the C-2 benzoyl substituent of taxol with a m-aminobenzoyl group (2-AB-taxol; Figure 1). It was hoped that such a minor modification of the parent molecule would not adversely affect the interaction of the analog with microtubules. Furthermore, it has been proposed that the substituent at the C-2 position is in contact with the protein when taxol is bound to the microtubule. An environmentally sensitive fluorescent probe, such as the aminobenzoyl group on 2-AB-taxol, should be amenible to testing this hypothesis.

Fluorescent Properties of 2-AB-Taxol
2-AB-Taxol is weakly fluorescent in aqueous solution. The quantum yield and
emission maximum of 2-AB-taxol increases with decreasing solvent polarity,
demonstrating that the fluorophore is capable of probing the local environment
of the ligand (Figure 2).
The emission maximum as a function of solvent is
shown to reflect the bulk dielectric properties of the solvent, i.e., a general
solvent effect, rather than molecular properties of the solvent, i.e., a
specific solvent effect (Figure 3).
2-AB-Taxol Promotes in vitro Microtubule Assembly
The critical concentration of microtubule protein in the presence of 20
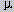 M 2-AB-taxol is
1.5
M 2-AB-taxol is
1.5 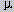 M compared to 0.3
M compared to 0.3
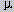 M for unmodified taxol
(Figure 4, A
and B). Thus, addition of an amino group meta to the ester decreases the
potency of the analog in this assay by about a factor of 5.
M for unmodified taxol
(Figure 4, A
and B). Thus, addition of an amino group meta to the ester decreases the
potency of the analog in this assay by about a factor of 5.
The extent of microtubule assembly, as judged by the relative increase in turbidity of the solution, increases with increasing 2-AB-taxol concentration up to a molar ratio of about 2:1 2-AB-taxol:tubulin (Figure 5). Similar experiments with taxol show that the extent of tubulin polymerization increases as a function of taxol concentration up to a maximum at 1:1 taxol:tubulin.[6] This result further demonstrates that 2-AB-taxol is less potent than taxol in promoting microtubule assembly.
2-AB-Taxol Promotes Normal Assembly of Tubulin into Microtubules
Electron micrographs of tubulin polymerized by 2-AB-taxol showed normal
microtubules (Figure 6).
2-AB-Taxol Bound to Microtubules Affects Tubulin Fluorophores
Energy transfer from the protein to 2-AB-taxol is observed in both the
excitation (Figure 8A) and emission
(Figure 8B) spectra of the polymer-bound
ligand. Protein emission when 2-AB-taxol is bound to microtubules is clearly
observed when emission at 450 nm is monitored
(Figure 8A). The emission
spectrum of microtubules liganded with 2-AB-taxol shows a slight shift to
higher energy and emission from 2-AB-taxol
(Figure 8B).
2-AB-Taxol Binds to the Colchicine-Tubulin Complex in the Monomeric State
Although it is widely thought that taxol does not bind to unpolymerized tubulin,
it has been shown previously that taxol enhances tubulin GTPase activity
induced by colchicine.[7] These data indicate that the taxol binding site may be
wholly contained in the tubulin monomer and would not necessarily need to span
more than one monomer in the protein. In
Figure 9 it is shown that the emission
spectrum of 2-AB-taxol shows a shift to higher energy in the presence of the
tubulin-colchicine complex. No turbidity of the solution could be observed by
absorption spectroscopy, and an electron micrograph of the solution showed no
microtubules or other structures (data not shown).

The aromatic ring at C-2 on taxol appears to contact the protein in its complex with microtubules: (1) Addition of a meta amino group on this ring decreases the activity of the analog relative to taxol. (2) The emission spectrum of 2-AB-taxol bound to microtubules shows the fluorophore to be in a hydrophobic environment.
A portion of the taxol binding site has been previously identified to contain
the amino terminus of 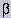 -tubulin.[8] The observation
that energy transfer from the
protein to the fluorophore in 2-AB-taxol suggests that at least one of the four
tryptophan residues of
-tubulin.[8] The observation
that energy transfer from the
protein to the fluorophore in 2-AB-taxol suggests that at least one of the four
tryptophan residues of 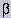 -tubulin is in close
proximity to the taxol binding site.
-tubulin is in close
proximity to the taxol binding site.
2-AB-taxol binds to the colchicine-tubulin complex in the monomeric state. Thus, the taxol binding site may be wholly contained on a single tubulin dimer.


2. Hamel, E. (1990) Interactions of tubulin with small ligands, in Microtubule Proteins (Avila, J., ed), pp. 89-191. CRC Press, Inc., Boca Raton, FL.
3. Horwitz, S. B. (1992) Mechanism of action of taxol. Trends Pharm. Sci. 13, 134-136.
4. Parness, J. and Horwitz, S. B. (1981) Taxol binds to polymerized tubulin in vitro. J. Cell Biol. 91, 479-487.
5. Chaudhary, A. G., Gharpure, M. M., Rimoldi, J. M., Chordia, M. D., Gunatilaka, A. A. L., Kingston, D. G. I., Grover, S., Lin, C. M., and Hamel, E. (1994) Inexpectedly facile hydrolysis of the 2-benzoate group of taxol and synthesis of analogs with increased activities. J. Am. Chem. Soc. 116, 4097-4098.
6. Diaz, J. F. and Andreu, J. M. (1993) Assembly of purified GDP-tubulin into microtubules induced by taxol and taxotere: reversibility, ligand stoichiometry, and competition.
7. Carlier, M.-F. and Pantaloni, D. (1983) Taxol effect on tubulin polymerization and associated guanosine 5'-triphosphate hydrolysis. Biochemistry 22, 4814-4822.
8. Rao, S., Krauss, N. E., Heerding, J. M., Swindell, C. S., Ringel, I., Orr,
G. A. and Horwitz, S. B. (1994) 3'-(p-azidobenzamido)taxol photolabels the
N-terminal 32 amino acids of  -tubulin.
J. Biol. Chem. 269, 3132-3134.
-tubulin.
J. Biol. Chem. 269, 3132-3134.
