CHEM 335 - NMR Laboratory Tour
Vintage (1970s) NMR system: 60 MHz (B0 = 1.41 Tesla = 14100 Gauss)
(Continuous Wave NMR with Electromagnet)
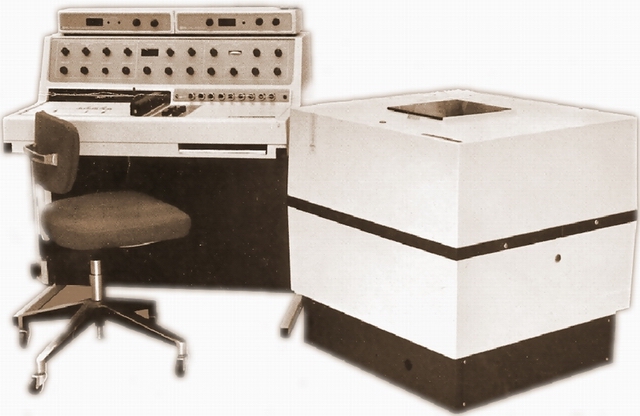
A spectrometer similar to this Varian EM-360A was in use in our department from 1979 until 1995.
It observes protons at a frequency of 60 MHz in a magnetic field of 1.41 Tesla.
For comparison: Today's benchtop NMRs are much more compact and user-friendly.
These instruments use Rare Earth Permanent Magnets with fields up to 2.35 Tesla.
Primary applications are undergraduate teaching and industry process control.

|

|

|
However, most research samples require much stronger fields of 7 Tesla or higher for improved resolution and sensitivity.
Uniform magnetic fields of such strength can currently only be achieved with superconducting magnets (present limit: 28 Tesla).
BU's Chemistry Department has been operating several NMR spectrometers up to 600 MHz (B0 = 14.1 Tesla).
Vintage (1980s) NMR system: 100 MHz (B0 = 2.35 T)
(Pulse / Fourier Transform NMR with Superconducting Magnet)

This Bruker AC-100 NMR spectrometer and similar AC-300 and AM-360 instruments
Modern NMR systems: 600 MHz (B0 = 14.09 T) and 400 MHz (B0 = 9.41 T)
Our most recent acquisitions in the Smart Energy Building are a Bruker Avance III 600 and a Bruker NEO 400.
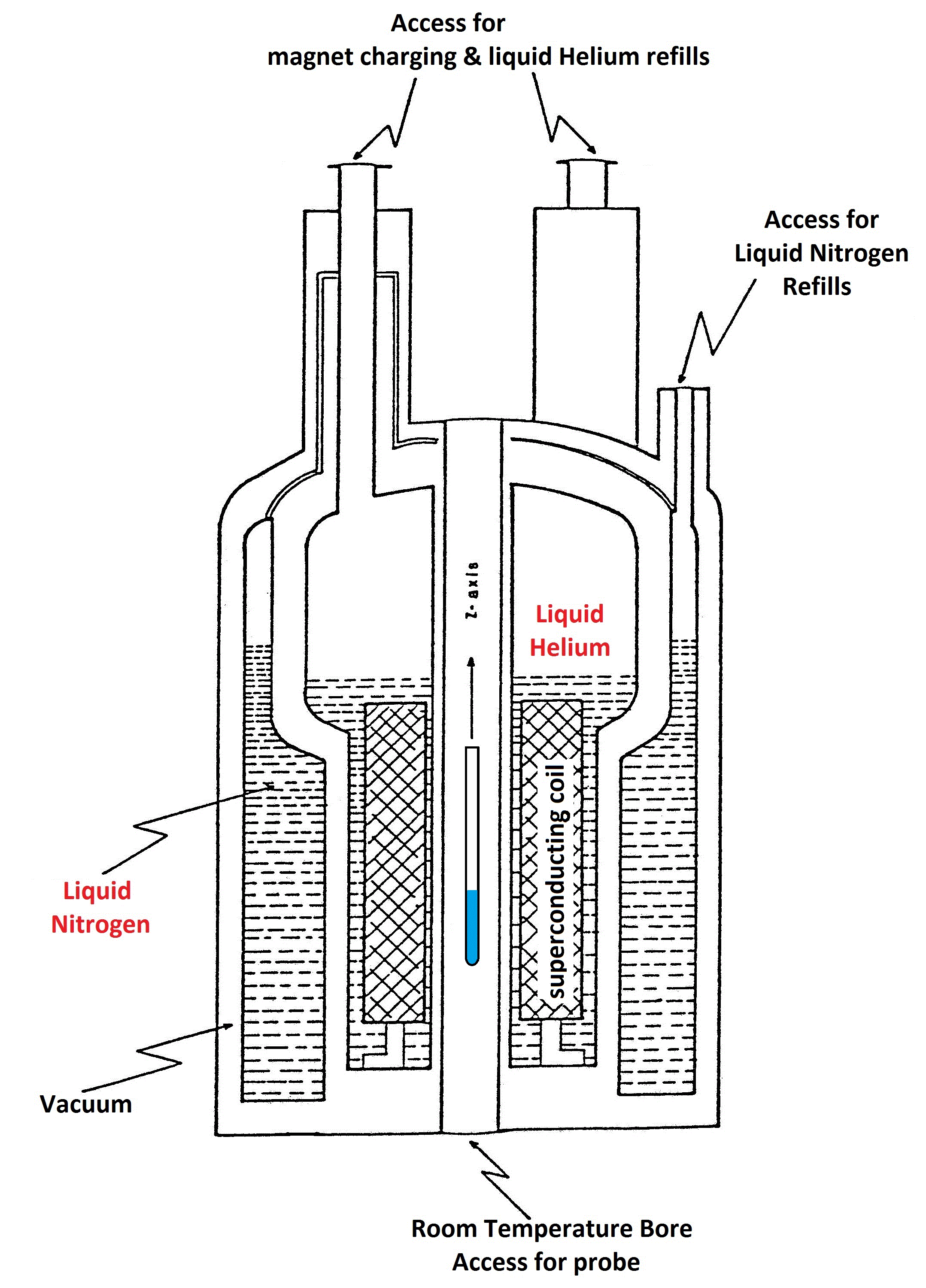
Schematic and Inside View of Superconducting NMR Magnets.
The innermost container holds the superconducting coil in a bath of liquid Helium.
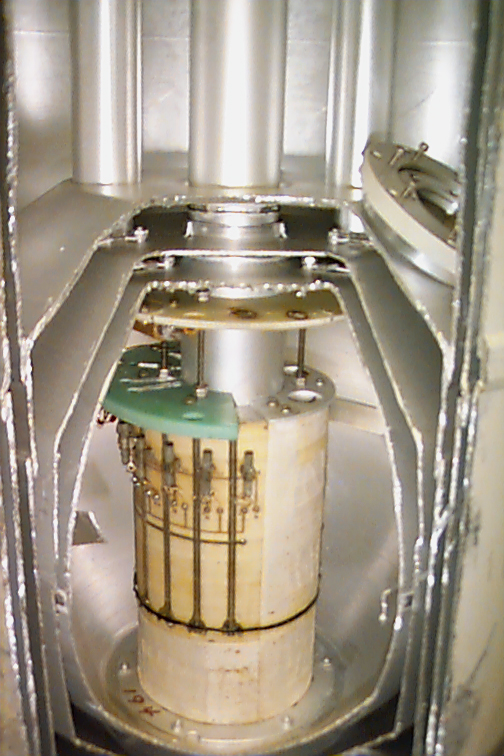
Superconducting NMR Magnet during Helium Refill
Periodic cryogen refills are necessary to keep the magnet in operation.
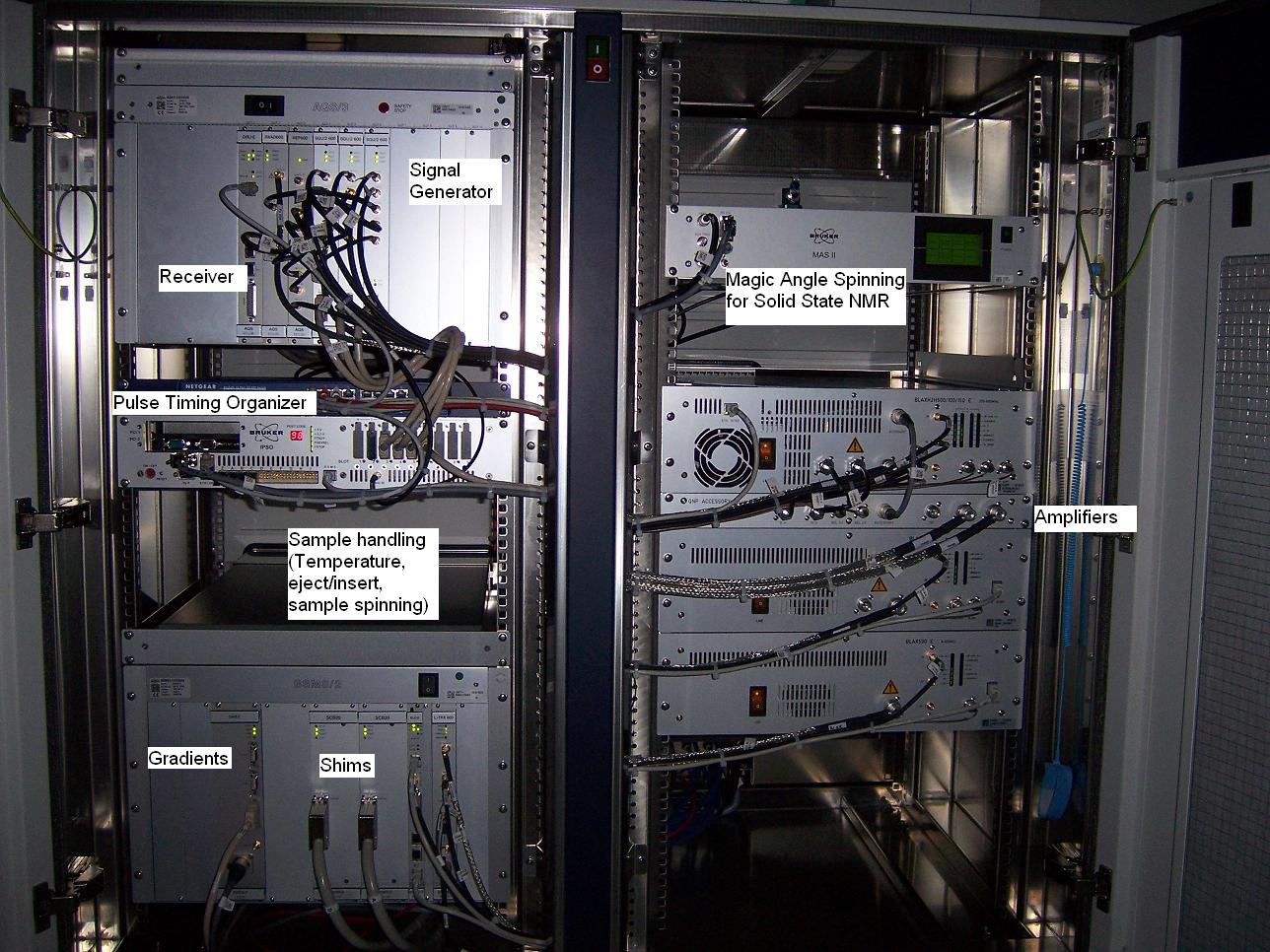
The inside of a modern NMR Console with essential components highlighted.
RF pulses from the signal generators are amplified and sent to the probe (see below) to excite specific nuclei, i.e. 1H or 13C in the sample.
NMR Probes
The probe, which is located in the center of the magnet, detects signals from the excited nuclei and sends them back to the receiver.
The NMR Experiment - Sample Preparation
NMR sample tubes are filled with 0.5 ml of a solution of a compound of interest.
The NMR Experiment - Sample Change
The sample tube is placed into a sample holder ("Spinner") and inserted into the magnet.
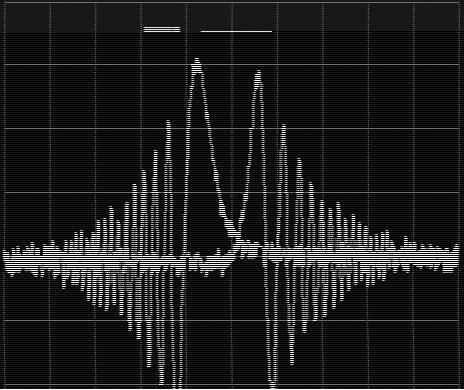
The NMR Experiment - Deuterium NMR Signal for Field/Frequency Lock
The 2H atom in the deuterated solvent (i.e.: C6D6 or D2O) is observed via a separate NMR experiment.
Unlocked Sweep Signal vs. Shimmed Lock Signal
The NMR Experiment - Data Acquisition
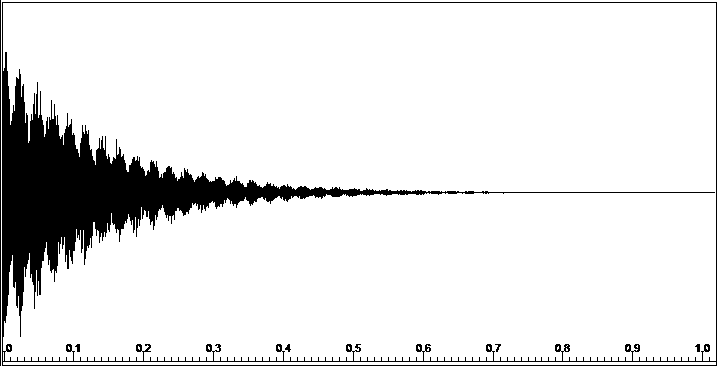
A 90o RF Pulse generates a Free Induction Decay (FID), which is recorded during the aqcuisition time ("The Scan").
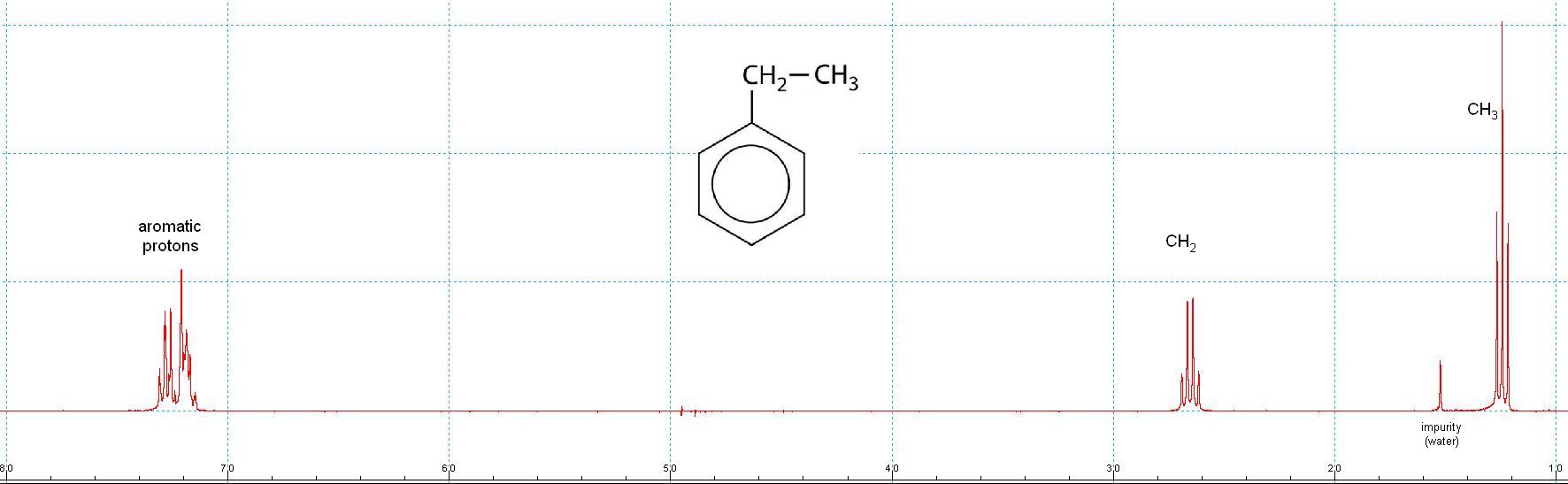
300 MHz Proton NMR Spectrum of Ethyl Benzene after Fourier Transformation of the FID.
75 MHz 13C NMR Spectra of Ethyl Benzene
top: 1H coupled: Multiplicities due to 1JC,H couplings allow the identification of CH3, CH2, CH, and Cq signals.
Bigger molecules have more complicated (more interesting) spectra.
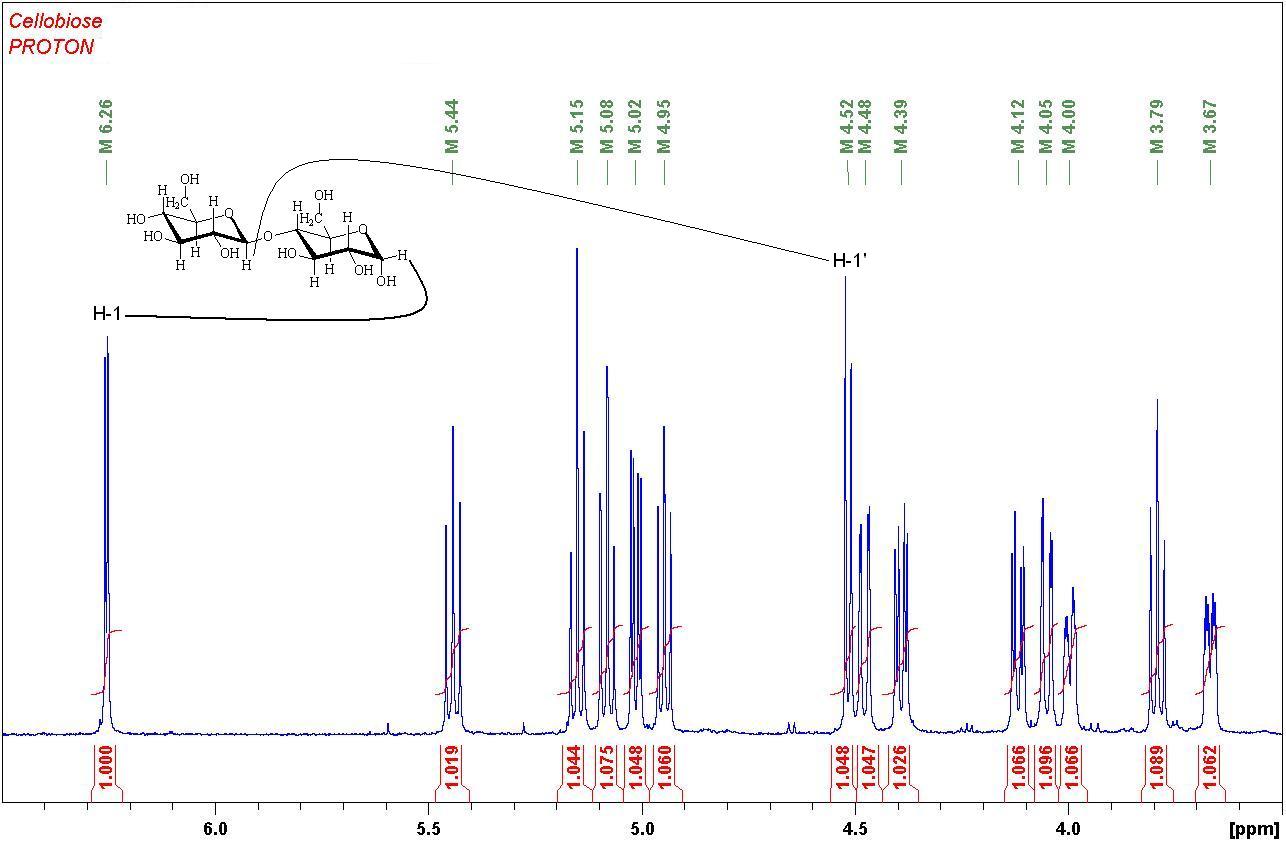
1H NMR Spectrum of the disaccharide Cellobiose
To fully characterize the structure, two-dimensional NMR techniques have to be employed:
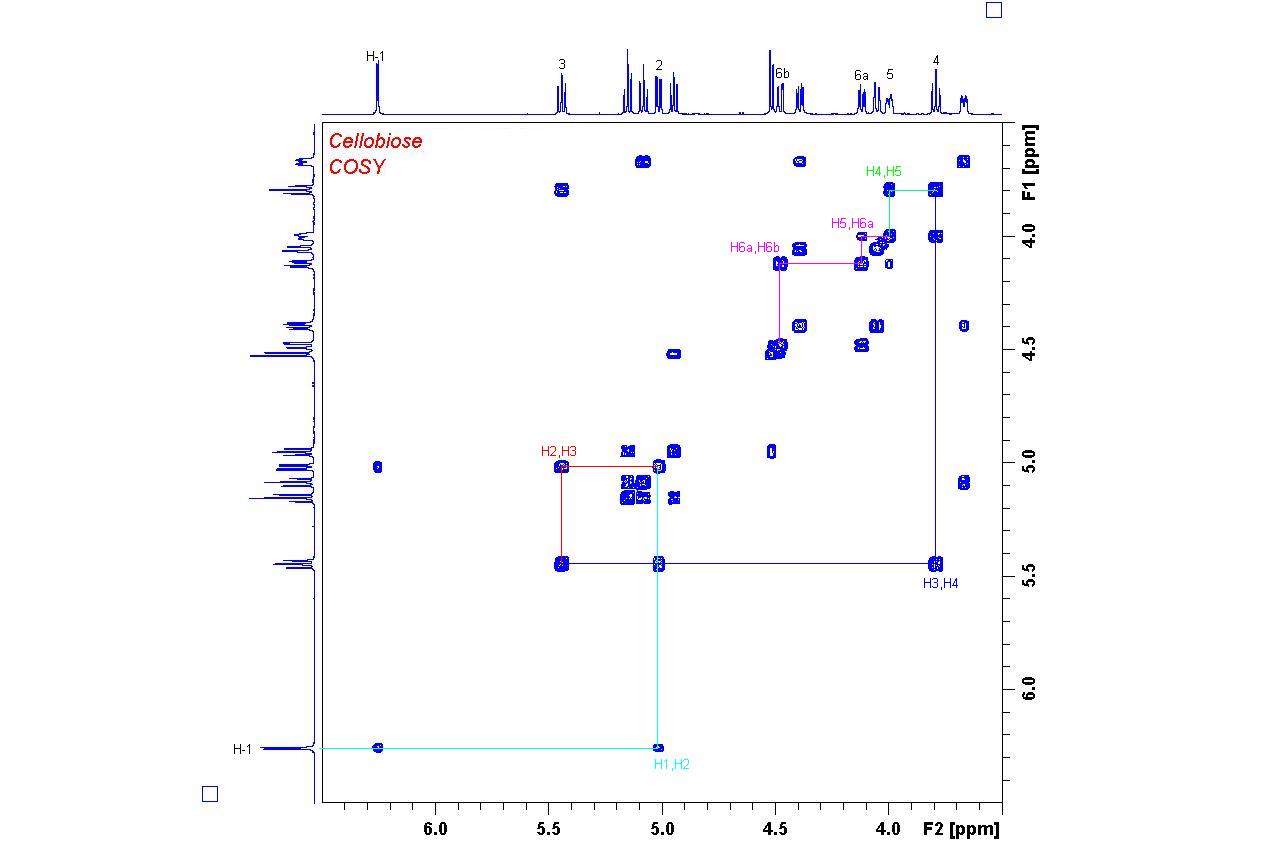
1H,1H COSY Spectrum of Cellobiose
Once all proton signals have been assigned, carbon signals can be found through a C,H correlation experiment:
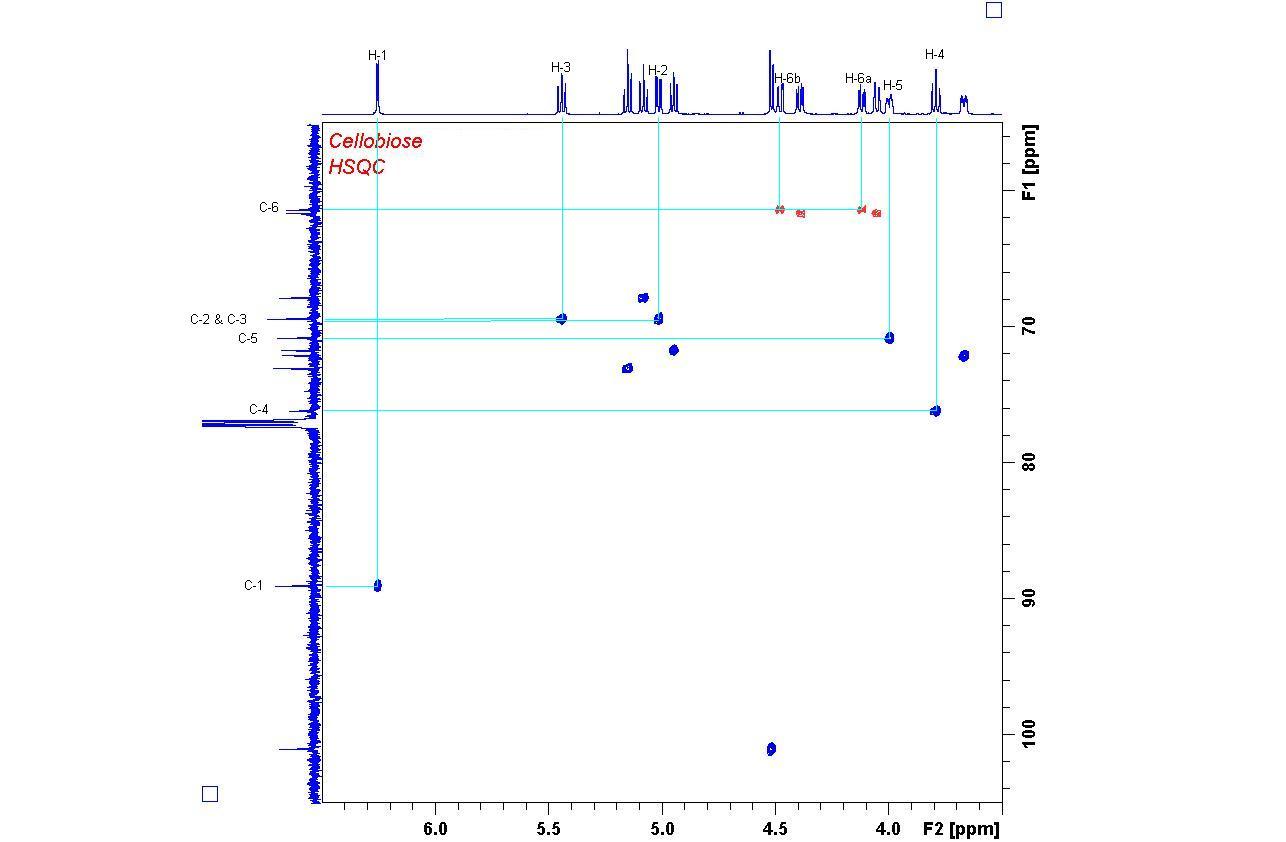
1H,13C HSQC Spectrum of Cellobiose
(operating at proton resonance frequencies of 100, 300, and 360 MHz, respectively)
were in service in our department until December 2022 and were used for research and by our instructional labs.

They are primarily used for the structure determination of organic and biochemical samples in solution and in solid state.
Surrounded by several vacuum layers with liquid Nitrogen between them, the Helium loss is minimized to 10-20 liters per month.


Gradients separate wanted from unwanted signals. Shims make the magnetic field uniform to optimize signal resolution and sensitivity.
Probes usually have more than one coil to be able to manipulate several nuclei (i.e.: 1H, 13C, 15N, 31P) simultaneously.
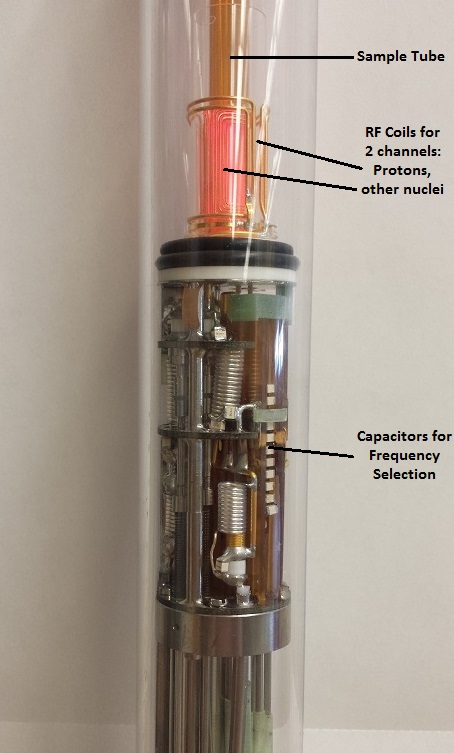

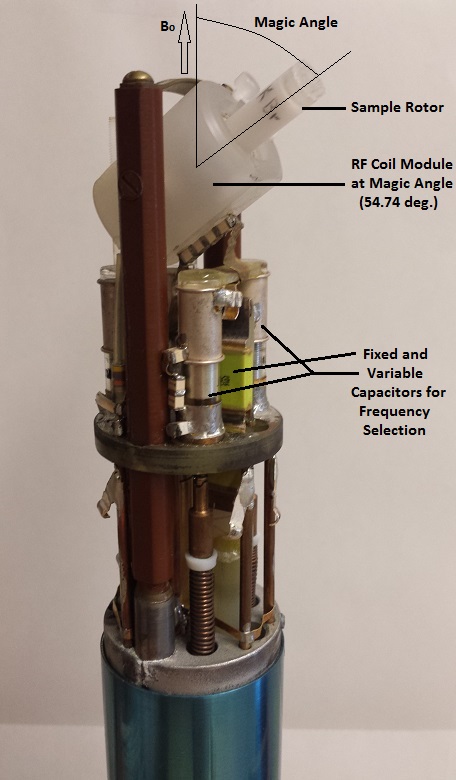
left: Probe for Solution NMR
right: Probe for Solid State NMR
The use of deuterated solvents minimizes solvent signals. Tetramethylsilane (TMS) may be added as a reference compound.


Click the picture to view the video.
It allows us to monitor the stability of the magnetic field and to improve the field homogeneity ("Shimming").
Repeated scanning will improve the signal/noise ratio (Signal Averaging) and is essential for the direct observation of less abundant isotopes, i.e.: 13C or 15N.
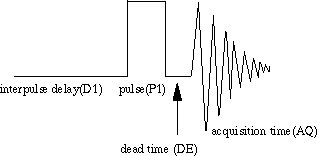
Different protons resonate at different frequencies (=> chemical shifts).
Proton signals often are split into multiplets, indicating couplings with other protons.
bottom: 1H decoupled: Proton decoupling simplifies the spectrum by collapsing each multiplet into one single peak.
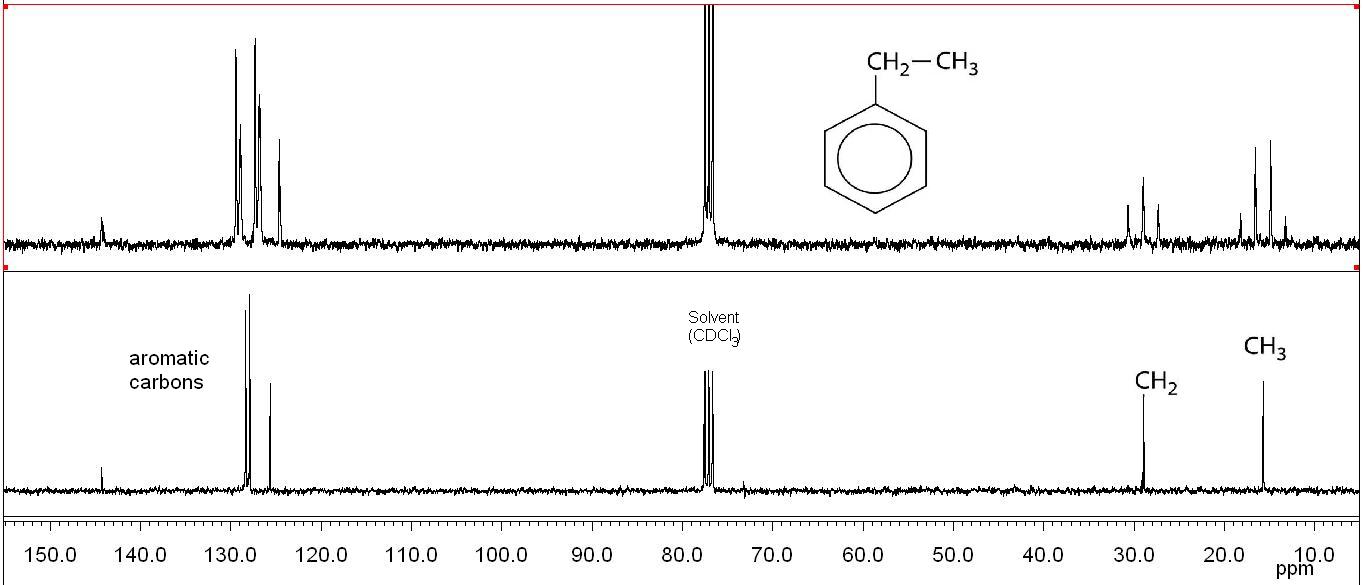
Integration of the individual proton signals confirms the presence of 14 H atoms on the rings.
The H-1 signals for each ring are easily identified as doublets, as each is only coupling to one proton.
All other protons are coupling with more than one proton and show more complicated multiplicities.
Starting from proton H-1 at 6.26 ppm, all other protons can be found by following the crosspeak patterns.
The connectivities for one of the two monosaccharide units have been drawn into the spectrum.
Try finding the connections in the other ring by starting from the H-1' doublet at 4.52 ppm.
The HSQC spectrum allows the immediate identification of direct Carbon-Proton bonds (1J coupling).
Note the negative CH2 signals (red) which are easily distinguishable from CH and CH3 (blue).