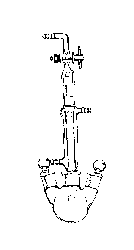
Synthesis of W(CO)4(bipy):
2,2'-bipyridinetetracarbonyltungsten(0)
Introduction
The air-stable complex 2,2-bipyridinetetracarbonyltungsten(0) may be effectively prepared in solution and under an inert atmosphere by the displacement of two monodentate carbonyl ligands with one bidentate 2,2-bipyridyl (bipy) ligand. In solution, it is almost impossible to replace two additional carbonyl ligands with another bipy ligand. Although bipy is a s-donor/p-acceptor ligand, it is not as strong a p-acceptor as other ligands, such as diphosphine [(CH2.PR2)2]. This fact conveniently limits the displacement of carbonyl ligands by bipy to two.
A number of different synthetic methods lead to 2,2-bipyridinetetracarbonyltungsten(0). One method involves the photolysis of a solution of the metal hexacarbonyl and 2,2-bipyridine.
Experimental1,2
CAUTION: Metal carbonlys and xylenes are toxic compounds and should be handled with care. Avoid contact, use gloves and work in a fumehood.
Approximately 60 mL of xylene solvent (boiling point 137-144 °C) is placed in a 250 mL three-neck round bottom flask (ground joint size 24/40), equipped with a small magnetic stir bar. One neck of the three-neck flask is stoppered and the center neck is fitted with a condenser equipped with a gas inlet (Figure 1). All joints are lightly greased with vacuum grease and secured with green Keck clips. Split the nitrogen line so the flow rate can be monitored with a bubbler, and purge the flask.
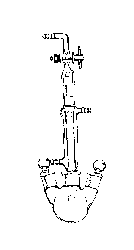
Figure 1. A reflux apparatus is used for the synthesis of the transition metal complex.
Using a positive flow of nitrogen, tungsten hexacarbonyl (0.85 g) and 2,2-bipyridine (0.40 g) are added, with stirring, via a powder funnel inserted in one neck of the flask. Remove the funnel and cover the neck of the flask with a rubber septum. The reaction mixture is refluxed for two hours under nitrogen (split the nitrogen line to attach to the other students' flasks, if applicable, as well as to the inlet of a bubbler). Upon cooling, a deep purple solid forms. Remove the heating mantle and place the flask in an ice bath to help cool the product. This solid is vacuum filtered, washing with ~25 mL hexanes. Place the product and filter paper on a watch glass or Petri dish and leave to dry until the next lab period.
Characterization and Laboratory Report
Include balanced equations for all reactions performed. Report the percent yield of the complex. Infrared spectroscopy of the metal carbonyl complex is to be performed in a saturated chloroform solution using a 1.00 mm pathlength solution IR cell; alternatively nujol mulls may be prepared. Report and discuss the carbonyl stretches. Using basic group theory, and the support of literature,3 do these stretches indicate a cis- or trans- bipy ligand? Assign the absorption bands to the vibrations of the carbonyl ligands. How could one change the complex that would in turn alter the number of carbonyl stretches or carbon-oxygen bond order?
The UV-vis spectrum (see Appendix) of 2,2-bipyridinetetracarbonyltungsten(0) displays a solvent- dependence. Prepare three samples that are 8 x 10-5 M concentration in each of the three solvents: dimethylformamide (DMF), tetrahydrofuran (THF) and diethyl ether. What do you observe? Also record UV-vis spectra of the three solutions. Explain the spectral similarities and differences. Assign the absorption bands and discuss any MLCT or ligand field effects.
CAChe
Using the CAChe molecular modeling software, construct the metal carbonyl complex. Can you construct a trans- and a cis- bipy complex? Optimize the structure using Mechanics. Are the metal-nitrogen bond lengths reasonable in each complex? Are the bond angles and total energy reasonable? Does the result of your modeling efforts support your assignment of the molecular structure, based upon infrared spectroscopy?
Include copies of spectra (label axes and give titles) and all CAChe work with your report.
References
1. Manuta, D. M.; Lees, A. J. J. Chem. Ed. 1987, 64, 637-638.
2. Stiddard, M. H. B. J. Chem. Soc., Dalton Trans. 1962, 4712-4715.
3. Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed., John Wiley: New York, 1986, pp. 269-272. On hold for Chem445 in Science Library. (Science Library, Call Number: QD96 .I5N33 1986)
Additional Reading
1. Elschenbroich, C.; Salzer, A. Organometallics: A Concise Introduction; Second Edition; VCH: New York, 1992; pp. 220-251. (Science Library, Call Number: QD411.E4413 1992)
2. Dickson R. S.; Elmes, P.S.; Jackson, W.R. Organometallics 1999, 18, 2912-2914.