Metallocene and its Derivatives: Synthesis,
Properties and Electrochemistry
Introduction
The cyclopentadienyl ligand (Cp) is one of the most common ligands in transition metal complex chemistry, after the carbonyl group. The symmetrical 5-carbon ring coordinates to one face of transition metal octahedra, to define an h5 (pronounced "eta-five") coordination complex. This coordination is characterized as a p-bonding interaction, with the ligand treated as a 6-electron donating anion (C5H5-), or a 5-electron donating neutral substituent. Cyclopentadiene can also be treated as a 1-electron donor in a bonding situation known as monohapto.
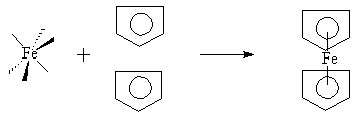
The structure of a metallocene complex, if cyclopentadiene is the only coordinating ligand, is a transition metal sandwiched between two Cp rings. One common example of such a complex is the coordination of Iron (II) to form ferrocene (Fc) (Figure 5.1). Ferrocene is a diamagnetic orange solid that can be readily purified by sublimation at 100°C, is stable in air, and is insoluble in water.

Figure 1. Ferrocene has a sandwich structure, where the Cp rings are
staggered or eclipsed; this example displays the latter.
The chemistry of ferrocene is extensive. Primarily, this chemistry involves Friedyl-Crafts substitution of the Cp rings. In fact, the rate of these substitutions has been shown to exceed the reactivity of benzene by a factor of 106. In addition, the electronic structure of ferrocene makes it very stable with respect to oxidation. The one-electron oxidation product of ferrocene is called ferrocenium (Fc+), and is most easily prepared by bulk electrochemical methods. Unlike many transition metal oxidation products, ferrocenium is relatively stable in air, making it useful as a mild chemical oxidant. The Fc/Fc+ couple is also extensively used as a reversible electrochemical standard.
In this experiment, we will characterize this complex by melting point determination, UV-vis and FTIR spectroscopies, as well as cyclic voltammetry. We will perform a bulk oxidation of ferrocene and characterize the ferrocenium oxidation product. Finally, a classic aromatic electrophilic substitution reaction will be used to acetylate ferrocene. The monoacetylferrocene derivative is the primary product of this reaction, and will be purified using column chromatography and recrystallization. Finally, we will use CAChe molecular modeling software to investigate the electronic and vibrational spectra of these iron based metallocenes, and to design substituted ferrocene molecules with new properties.
Experimental
Purification of Ferrocene
The solid is spread out on a large
watch glass and sublimed from a large glass petri dish that is placed on a warm hot plate
(100°C). Care is used to avoid charring the ferrocene. The
sublimed product is collected over the watch glass on a cold finger.
Preparation of Acetylferrocene
A mixture of 1.5 g of ferrocene and 5.0 mL of acetic anhydride (extremely corrosive; lachrymator; wear gloves) is prepared in a small Erlenmeyer flask. 1.0 mL of 85% H3PO4 (extremely corrosive; wear gloves) is added dropwise, with constant stirring, to this mixture. This addition is exothermic and accompanied by a change in color. After all the phosphoric acid has been added, the Erlenmeyer flask is fitted with a CaCl2 drying tube, and the solution is heated to 50°C in a water bath for ten minutes. The mixture is then poured over 20 g of ice into a large beaker that will accommodate the gas that will be formed in the following steps. Water is used to rinse the reaction flask and maximize product yield.
When the ice has melted, small quantities of sodium bicarbonate (NaHCO3) are added until gas evolution stops. The pH may be tested with pH paper or a pH meter, to insure that neutrality is achieved. Cool the beaker in an ice bath for 30 minutes, and a precipitate should form. Suction filtrate the dark brown product using a coarse fritted funnel, and wash with distilled water (~ 1500 mL) to remove impurities. Allow the pale-orange solid to air-dry for 15 minutes.
Thin layer chromatography is used to optimize the conditions for column chromatography. A silica gel slurry is made by adding 40 g of silica gel to 100 mL of chloroform. Five microscope slides are used as TLC plates; alternately, pre-made TLC plates may be supplied. A small amount of the crude acetylferrocene is placed in a vial and dissolved in 2 or 3 drops of toluene. Do the same for a small amount of ferrocene. A line is penciled on each slide, approximately 1 cm from the bottom. The slides are spotted, approximately on the line, using a fine capillary applicator, for both solutions. Allow the spots to air dry and then apply a second spot, to obtain a more concentrated area of compound. The identity of the spot is indicated with a pencil mark. Repeat this procedure for the other four slides.
Place one slide, with the spotted end submerged in the solvent, into a developing chamber containing one of the following solutions: petroleum ether, toluene, ethyl ether, ethyl acetate and a mixture of 10% ethyl acetate and 90% petroleum ether. The pencil mark should be above the solvent level. The solvent containers are covered while the slides are developing. The slides are removed when the solvent front has traveled approximately 75% up the length of the slide. Mark the spots on the slides and allow the plates to air dry; for TLC slides, develop in an iodine chamber and mark the resulting brown spots.
The solutions that provide maximum solubility of the two components, and hence separation, are chosen as column chromatography solutions. For instance, ferrocene may elute with toluene, while the acetylferrocene remains on the column and is eluted with a toluene/ethyl acetate mixture. The color of the spots is helpful to discern the individual bands that elute from the column. The crude acetylferrocene is dissolved in the solution that is selected to elute the first component.
The column, a 50 mL burette, is assembled by placing a small piece of glass wool
into the bottom of the column. The glass wool is then covered
with a small amount of sand and the burette is filled with the non-polar
solvent that was chosen to dissolve the crude mixture. A powder funnel
is used to slowly fill the column with silica gel, to a height of approximately
30 cm. The column is never allowed to dry. A small amount of silica gel
may be added to the crude acetylferrocene solution to make a slurry. Then
add the slurry to the top of the column and cover with a small amount
of sand. The two solutions (or mixtures) are then used to purify the crude
acetylferrocene. Gradually add a small amount of the polar solvent to the
non-polar solvent until the acetylferrocene begins to elute. The ferrocene
band (first band) is discarded and the solvent is removed from the acetylferrocene
band (second band) by rotary evaporation. The acetylferrocence may then be recrystallized
from chloroform.
Cyclic Voltammetry
Prepare 100 mL of a 0.1 M solution of tetrabutylammonium hexafluorophosphate (TBAH) (recrystallized from hot ethanol) in acetonitrile. This solution will provide an excess of supporting electrolyte. Assemble an electrochemical cell in a small beaker (10 mL) equipped with a septum and small magnetic stirring bar (Figure 4) . Use the potentiostat, a platinum button working electrode, a platinum wire (or platinum paddle) counter electrode and a freshly prepared Ag/AgCl reference electrode.
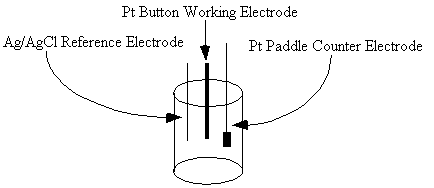
Figure 2. The electrochemical cell contains three elctrodes.
Obtain a background limit in the range -1.0 to +1.0 V (current range ~ 10 mA) using the cell, bubbling the TBAH/acetonitrile solution with nitrogen prior to adding either ferrocene or acetylferrocene (approximately 3.2 x 10-3 M, current range 10-100 mA). Typical scan rates are 100-500 mV/sec. The platinum wire or paddle may be cleaned prior to use by placing it in the flame of a Bunsen burner. The platinum button electrode is cleaned using a BAS polishing kit. The platinum electrodes must be cleaned prior to each cyclic voltammetry experiment. Clean electrodes and glassware are essential to accurate cyclic voltammograms.
To prepare the Ag/AgCl reference electrode, a one volt potential is
applied across a cell that consists of a silver wire (which has been soaked
in a concentrated sulfuric acid solution and rinsed in water) and a platinum
wire or paddle. The two wires are placed in a 6 M HCl solution. The reference
and counter electrode cables are connected to the platinum wire. The working
electrode cable is connected to the silver wire. Caution: do not touch
the wires during the preparation of the AgCl reference electrode.
Preparation of Ferrocenium
Using the potentiostat, a reticulated vitreous carbon (RVC) working electrode, an Ag/AgCl reference electrode, and a fritted thimble equipped with a platinized titanium mesh counter electrode, perform a bulk oxidation of ferrocene (~ 4 x 10-3 M) in the TBAH/CH3CN (0.1 M) solution. The dilute solution of ferrocene may be prepared by the addition of ~ 0.045 g ferrocene to 60 mL of degassed acetonitrile/TBAH solution in the electrochemical cell (be sure to adjust your solution level and ferrocene quantity when the cell is assembled). It is not necessary to obtain a background limit prior to conducting the bulk electrolysis. The optimum conditions for the bulk electrolysis are achieved by maintaining a potential of 450-550 mV, for 2 to 3 cycles of 200 seconds. The current should be in the 1.5 x 10-2 A range. If the solution is not blue, the potential can be increased. The current range is set on the potentiostat to 10 mA. When the solution is blue, the electrolysis is stopped and a UV-Vis absorption spectrum of the blue solution is obtained immediately.
Characterization and Write-up
Ferrocene and acetylferrocene should be characterized by melting point determination, IR and UV-Vis spectroscopies in acetonitrile solution, and by cyclic voltammetry. In each case, compare the experimental results of the two complexes in terms of their electronic structure. Explain any differences in the observed spectroscopy. Compare your melting point, IR and UV-vis experimental results with results reported in the literature.
Finally, the CAChe molecular modeling software package can be used to construct and minimize the structure of each complex (see Appendices). Note that only two structures, eclipsed and staggered, are drawn or imported from the fragment library: since the atoms remain the same, only the number of electrons or the substituent changes. What are the most notable similarities and differences of the modeled ferrocenes? How do the eclipsed and staggered molecules compare? Compare a ferrocene and a ferrocenium molecule, as well as a ferrocene and an acetylferrocene molecule. Are the carbon-iron distances affected when the staggered ferrocene molecule is oxidized to the ferrocenium cation? A project leader table may be helpful for these comparisons. How do the calculated results compare with your theoretical expectations?
How do the results of the molecular modeling compare with the observed spectroscopy (i.e., how do the calculated UV-vis spectra compare with the colors that you experimentally observe)? Select one molecule and determine the effect on the calculated UV-vis spectrum when you change the dielectric in the self consistent reaction field. The default is 78.5 (H2O). Generate one UV-vis in H2O and one in another solvent for comparison. Other solvents and their parameters are listed in the ZINDO manual. Use the software to simulate the electronic spectroscopy, and include both experimental and calculated plots in your final report. CAChe can also be used to remodel the ferrocene molecule to achieve a color change. A compound that achieves this goal should be proposed, including a potential method for its preparation. Keep in mind the standard substituents that you encountered in Organic Chemistry with the benzene molecule. They may be selected on the basis of electron donating or withdrawing ability to fulfill the task of your target molecule.
Your lab report should include eight structures that you have optimized using CAChe. This includes eclipsed and staggered ferrocene, acetylferrocene, ferrocenium cation and an individual target molecule. The report should also include UV-vis spectra generated through ZINDO, and any project leader tables that support your discussion.
References
1. Angelici, R. J., Synthesis and Techniques in Inorganic Chemistry, 2nd ed., Saunders: Philadelphia, 1977.
2. Bozak, R. E. J. Chem. Educ. 1966, 43, 73.
3. Haworth, D. T.; Liu, T. J. Chem. Educ. 1976, 53, 730.
4. Szafran, Z; Pike, R. M.; Singh, M. M., Microscale Inorganic Chemistry, Wiley: New York, 1991.
5. Jolly, W. L., The Synthesis and Characterization of Inorganic Compounds, Prentice- Hall, New Jersey, 1970.
6. Bard, A. J.; Faulkner, L. R., Electrochemical Methods: Fundamentals
and Applications, John Wiley and Sons: New York, 1980.