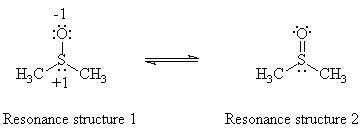
Transition Metal Complexes of Dimethyl Sulfoxide:
The Preparation of Cu(DMSO)2Cl2 and Ru(DMSO)4Cl2
Introduction
The nature of dimethyl sulfoxide (DMSO, (CH3)2SO) as
a monodentate ligand is explored in this set of experiments.1,2
DMSO can ligate metal atoms by bonding in one of two
possible ways: through the oxygen atom
or through the sulfur atom (Figure 1). The method of bonding in the complex
can be determined with the use of infrared spectroscopy, and the nature
of the ligand and its complexes can be explored using molecular modeling
software from CAChe Scientific (see appendix of this manual).
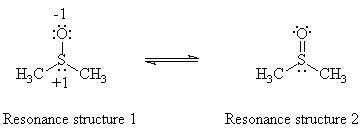
Figure 1. Resonance structures of DMSO.
The infrared spectrum of dimethyl sulfoxide shows an absorption peak at 1050 cm-1, which arises from the sulfur-oxygen bond. If a Lewis acid bonds to the sulfur atom, the contribution of resonance structure 1 decreases, and the SO stretching frequency increases. Conversely, if a Lewis acid bonds to the oxygen atom, the contribution of resonance structure 2 decreases, and the SO stretching frequency decreases.3
Experimental
The preparation of Cu(DMSO)2Cl2 (approximate time required: 30 minutes)
300 mg of copper(II) chloride is placed into a 25 mL Erlenmeyer flask equipped with a magnetic stirring bar. A calibrated Pasteur pipet or syringe is used to add 2.0 mL of absolute ethanol to the flask. The mixture is stirred until all of the copper(II) chloride has dissolved. 0.5 mL of DMSO is then added slowly with an automatic deliver pipet or syringe. An immediate exothermic reaction will occur, yielding a light green precipitate. The mixture should be stirred for five minutes.
The product is collected by suction filtration using a Hirsch funnel.
The crystals should be washed with cold ethanol (two 1.0 mL portions) and
air-dried on a piece of filter paper placed on a watch glass. The crystals
can then be weighed for a percent yield calculation. Determine the melting
point and obtain an infrared spectrum of the product in chloroform
solution. If the product is insoluble in chloroform, prepare a Nujol
mull. Also obtain an infrared
spectrum of DMSO. For your report, determine the absorption frequency of the S-O
band for both the complex and DMSO solvent.
The preparation of Ru(DMSO)4Cl2 (approximate time required: 2.5 hours)
0.30 g RuCl3·3H2O is placed in a 10 mL round-bottomed flask equipped with a magnetic stirring bar. A water condenser is attached to the flask and the apparatus is placed on a heating mantle set on a magnetic stirring hot plate. Bubble nitrogen gas through 1.0 mL of DMSO placed in a graduated cylinder. Add the degassed DMSO to the round-bottomed flask, and heat to reflux with continuous stirring. Allow the mixture to boil for five minutes only. Do not overheat. The mixture will turn an brown-orange color when the reaction is complete. The solution is cooled and transferred, using a Pasteur filter pipet, to a 25 mL Erlenmeyer flask. Yellow crystals may separate out at this point; if this is the case, the crystals can be collected as stated below. Otherwise, the volume of the solution is reduced to approximately 0.5 mL by passing a gentle stream of nitrogen gas over the warmed liquid.
To isolate the product, 20.0 mL of dry, reagent grade acetone is slowly added to form two phases. The mixture is cooled in an ice bath. Yellow crystals should form upon standing for 10-15 minutes. Collect the crystals by suction filtration using a Hirsch funnel. The crystals are then washed with 500 mL of acetone , followed by 500 mL diethyl ether. The crystals are then weighed to calculate a percent yield. Determine the melting point and obtain an infrared spectrum to locate the absorption frequency of the S-O band.
Discussion
Compare the three infrared spectra of DMSO, Cu(DMSO)2Cl2 and Ru(DMSO)4Cl2 that you obtained, to determine the type(s) of bonding that occurs for the DMSO ligands to the corresponding metal atom. You should observe one band for the copper-DMSO complex and two bands for the ruthenium-DMSO complex (Figure 2). In your report, you should compare the experimental data to theoretical CAChe data (see below), as well as literature data available in the library.3
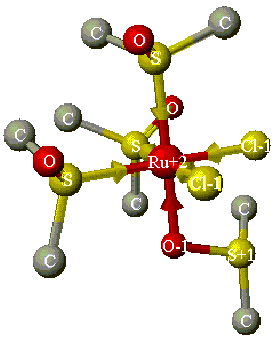
Figure 2. Structure of Ru(DMSO)4Cl2 (without
H atoms), minimized using Mechanics in CAChe. Note that three DMSO molecules are
S-bonded (coordinate covalent bonds indicated by arrows), while one is O-bonded.4
CAChe Component
The CAChe molecular modeling system allows one to investigate the DMSO ligand and the transition metal complexes that it can form. The software allows the construction and optimization of structures and the generation of theoretical infrared spectra. The general approach is as follows:
References
1. This experiment was adapted from Microscale Inorganic Chemistry, by Z. Szafran, R. M. Pike, and M. M. Singh, John Wiley: New York, 1991 and from a laboratory experiment developed by John C. Kotz of SUNY at Oneonta.
2. Reynolds, W. R. "Dimethyl Sulfoxide in Inorganic Chemistry", Progress in Inorganic Chemistry, S. J. Lippard, Ed., Interscience: New York, 1970, volume 12, 1.
3. Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed., John Wiley: New York, 1986, pp. 269-272. On hold for Chem445 in Science Library. (Science Library, Call Number: QD96 .I5N33 1986)
4. Mercer, A.; Trotter, J. J. Chem. Soc. Dalton Trans. 1975, 2480.
5. See the section entitled, "Limitations in vibrational spectra generated by MOPAC," in the CAChe MOPAC Guide.