Intercalation Chemistry: Injection
of Lithium into Tungsten Trioxide
Introduction
Intercalation
is defined as the reversible insertion of a
guest species into a lamellar host structure with the maintenance of the
structural features of the host.1 The perturbation in geometric, chemical, electronic,
and optical properties of both the host and guest species can be phenomenal in
this type of reaction.1 Tungsten trioxide, WO3, is
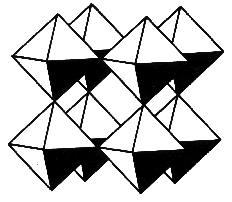
an open-framework material comprised of corner-sharing WO6 octahedra
(Figure 1). Group I metals can be intercalated into the void space
of the WO3 matrix, where the metal oxidizes and donates an electron
to the conduction band of WO3. This injection results in increased electron mobility, which
transforms the material from an electronic insulator to a conductor.2
A color change is also observed upon intercalation, resulting in a cubic
perovskite or tungsten bronze (general formula, ABX3).
(a) (b)


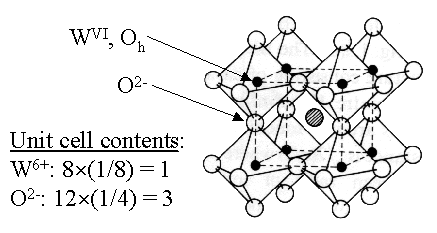
Figure 1. Cubic perovskite unit cell for MxWO3. (a) Block structure view. (b) Block structure view with atom centers shown. The sphere in the centre of the unit cell represents an intercalated cation.
LixWO3, 0 < x < 1, is an unstable material, and its sensitivity to air requires that it be produced under an inert atmosphere (N2) inside a dry box. Inherent thermodynamic restrictions of the lithium intercalation reaction require an effective reducing reagent, such as n-butyl lithium. N-butyl lithium essentially reduces the WO3 matrix, which provides the driving force for lithium intercalation between the negatively charged oxygens. The degree of intercalation of lithium atoms, x, is the crucial factor in determining the conductance of the resultant material. In order to determine this ratio, n-butyl lithium is used in excess. The unreacted n-butyl lithium will form LiOH upon reaction with isopropanol. The LiOH is titrated with standardized HCl to calculate the amount of lithium that was not intercalated into the initial tungsten trioxide sample.
Hydrogen intercalation of WO3
does not require an inert atmosphere. A redox reaction with zinc
metal provides an adequate driving force for hydrogen
insertion into the WO3 matrix.
Overview
The main objectives of this lab are to synthesize intercalated WO3 compounds and to evaluate changes in conductance that accompany these intercalation reactions. An ohmmeter will be used to evaluate the materials synthesized in this lab. The ohm is a measure of resistance. A decrease in resistance corresponds to an increase in conductance. As electrons gain mobility, the resistance of the material is reduced and the material is said to have gained metallic properties. Based on data obtained with an ohmmeter, one should be able to determine which materials underwent a successful intercalation reaction, i.e. the extent of cation insertion.
Using CAChe molecular modeling software, one will be able
to build the LiWO3 unit cell.
The space group for cubic perovskite is Pm3m.3 The value for a is 3.72 Å (a cubic structure requires that
a = b = c).4 The fractional coordinates
can be deduced from Figure 1, above. Compare your observations with those of the xylenes being driven
into the ZSM-5 zeolite. What are some of the possible complexities in the
lithium intercalation model?
Experimental
Reagents (for box--anhydrous):
n-Butyl lithium (1.6M in hexanes), hexanes, isopropanol, tungsten trioxide, (99 %+)
WO3, lithium iodide
Instruments and materials for dry box:
Ringstand, stir bars (2), scale, copper wire (18-gauge), glass capillary tubes (1.5 x 100 mm),
alligator clips (with electrical leads), vials (with Teflon caps), spatula
Reagents for bench-top:
Conc. HCl (12.1 M), std. NaOH (0.1000 M), phenolphthalein indicator, zinc metal
filings
Instruments and materials for bench-top:
Burette, separatory funnel, pipette, 500 mL volumetric flask, pH meter
Lithium Intercalation into WO3, Part A
This procedure takes place inside the dry box. 1.5 g WO3 is placed into a 250 mL Erlenmeyer flask, followed by 8.5 mL n-butyl lithium and 20 mL hexanes. An immediate color change of the tungsten trioxide is observed. The solution is stirred moderately and a stopper is fitted to the flask to prevent evaporation of the hexanes. After 48 hours, the reaction mixture is filtered through a glass frit into a clean 250 mL Erlenmeyer flask. The retained solid is washed three to four times with hexanes (approximately 15-20 mL aliquot for each wash). The hexanes filter at a very slow rate; therefore, the time between each washing should be spent standardizing the HCl and performing the hydrogen intercalation of WO3.
38 mL isopropanol is added to the excess n-butyl lithium/hexanes solution. The solution changes color. The frit is covered with aluminum foil and remains in the box (conductance measurements are performed on the dry solid). The flask is secured with a stopper and brought outside the box. Approximately 40-50 mL distilled water is slowly added to the solution. A white flocculent precipitate (LiOH) may be formed initially when a small amount of water has been added. When addition of the water is complete, a separation of the organic layer from the aqueous layer is observed and the white precipitate disappears.
Using a separatory
funnel, the layers are separated and the aqueous layer (bottom) is poured via a
glass funnel into a burette. A known volume of the LiOH solution is poured
into a flask and the solution is titrated with the standardized HCl until a pH of 7 is reached,
as measured with a pH meter or indicated by phenolphthalein indicator. If necessary, NaOH is available for back-titration.
Appropriate calculations are done to determine the number of moles LiOH (generated from excess n-butyl lithium and
isopropanol) that were present, based upon a 1:1 reaction of LiOH with HCl.
This amount is subtracted from the number of
moles lithium initially added (from n-butyl lithium), to give the actual number of
moles of Li intercalated. The value of x in LixWO3
may therefore be calculated, since the moles of WO3 starting material
is known.
Lithium Intercalation into WO3, Part A
Inside the dry box, 0.500 g WO3 is placed in a 250
mL Erlenmeyer flask. 25 mL hexanes and 0.800 g LiI are added and the
mixture is stirred. A stopper is fitted to the flask and the reaction is
left for 48 hours. The retained solid is separated from the filtrate with
a fritted funnel, as in part A. Conductance measurements are performed on
the dry solid.
Conductance/ Resistance Analysis
The conductance/ resistance measurements are taken inside the dry box. A straight piece of copper wire (approx. 18 gauge) is inserted into a 1.5-1.8 x 100 mm glass capillary tube (1/3 the length of the tube, Figure 2). The open end of the tube is placed into the WO3, and the tube then turned upside down, enabling the sample to fall to the inserted copper wire. This process is repeated until roughly 1/3 length of the tube contains well-packed sample. Another piece of copper wire is inserted into the open end and pressed securely against the sample. In order to avoid shattering the glass tube, one must straighten the copper wire with pliers and then slowly twist the wire into the tube. The wire is then bent at the ends of the tube to ensure a tightly packed tube.
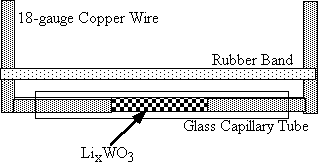
Figure 2. Apparatus used to measure the conductance
of solid materials.
A pair of alligator clips with electrical leads is used to connect the system above
to the outlet inside the dry box. Before the ohmmeter is connected to the
outlet outside the box, the instrument is calibrated by crossing the wires
until a reading of zero is obtained. Once the ohmmeter is connected to
the outside outlet, the instrument is turned on to 200 M Ohms. The scale
is decreased until a reading is obtained. Note: the ohmmeter is always
turned off after a series of measurements has been taken.
Standardization of HCl
A 0.1800 M stock solution of HCl is prepared in
a 500 mL volumetric flask. 10 mL of the stock solution is obtained with
a volumetric pipette and placed in an Erlenmeyer flask. The HCl is diluted
with water and 1 or 2 drops of phenolphthalein are added. This solution is then titrated
against standardized NaOH that has been placed in a 50 mL burette. Three
trials are performed to minimize error.
Hydrogen Intercalation into Tungsten Trioxide
0.50 g WO3 and 50 mL of 3M HCl are placed
in a 150 mL beaker. Less than 1 g of zinc metal filings are slowly
added to the beaker. Upon addition of the zinc, a reaction commences and
proceeds for approximately 1 hour (or until activity in the beaker ceases).
A notable change in color takes place with the WO3. The product is suction filtered and rinsed with distilled water.
The apparatus used to measure the resistance of both the
WO3 starting material and the HxWO3
intercalated material is the
same as for above (Figure 2), except the system is set up outside the box.
One
simply holds an ohmmeter wire to a copper wire at each end of the capillary
tube until an appropriate reading is obtained. The conductance for both materials is calculated as it was for LixWO3.
References
1. Whittingham, S. M. Intercalation Chemistry, Academic Press Inc.: London, 1982, p.1.
2. Whittingham, S. M. In Teaching General Chemistry: A Materials Science Companion; Ellis, Ed.; American Chemical Society; 1993; Experiment 8, ìHydrogen Insertion into WO3î.
3. Galasso, F. S. Structure, Properties and Preparation of Perovskite-Type Compounds , Pergamon Press: Hungary, 1969; p.7.
4. Ibid., p.23.
Additional References:
1. Shriver, D.F.; Atkins, P.; Langford, C.H. Inorganic Chemistry (Second Edition); W.H. Freeman and Co: New York, 1994; Ch.4.
2. West, Anthony R. Basic Solid State Chemistry; John Wiley and Sons Ltd.: 1988.