Preparation, Structure and Properties of
a High-Temperature Superconductor
Introduction
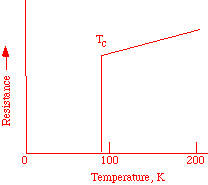
Onnes, a Dutch physicist, discovered in 1911 that mercury loses all resistance to electrical flow when cooled to approximately 4 K. A current, once started, will flow continuously and this phenomenon is known as superconductivity. At ordinary temperatures, metals have some resistance to the flow of electrons, due to the vibration of the atoms that scatter the electrons. As the temperature is lowered, the atoms vibrate less and the resistance declines smoothly, until a critical temperature, Tc, is reached. The value of this temperature depends on the material that is being cooled. At this point, the resistance drops abruptly to zero (Figure 1). If an electrical current is started in a superconducting ring, it will continue forever.

Figure 1. Electrical resistance of a superconductor.
Superconductors are also perfectly diamagnetic and repel a magnetic field. This property was discovered in 1933 and is known as the Meissner effect. A magnetic field induces a current in a conductor; conversely, a current induces a magnetic field. When a magnet approaches a superconductor, it induces a current in the superconductor. Since there is no resistance to the current, it continues to flow, thus inducing its own magnetic field which then repels the magnet’s field. If the magnet is sufficiently small and strong, the repulsion will be enough to counterbalance the pull of gravity and the magnet will levitate above the surface of the superconductor.
Until recently, the highest known critical temperature was 23 K, observed in the intermetallic compound Nb3Ge. Superconductors nonetheless found a number of applications, the most common being superconducting magnets for nuclear magnetic resonance (NMR) machines. NMR's are used extensively in research and for medical imaging. These instruments require liquid helium as the refrigerant, which is expensive. In a major breakthrough in 1986 (Nobel prize in 1987), Bednorz and Müller at IBM Zurich discovered superconductivity in copper-containing oxides at temperatures above 30 K, instigating a massive worldwide research effort. This culminated in the discovery of a metallic oxide of yttrium, barium, and copper that was superconducting at about 92 K. Liquid nitrogen (BP 77 K), which is cheaper and easily handled in, for example, a Dewar flask, could therefore be used as a coolant rather than helium. Related oxides now superconduct up to 133 K.
The compound, which has the formula YBa2Cu3O7-d, is readily prepared by heating an intimate mixture of yttrium oxide, barium peroxide, and cupric oxide at approximately 930°C for 10-12 hours. At this stage, the crystalline structure is formed by the interdiffusion of ions, but has an oxygen deficit (d ~ 0.5). Cooling the material to 500°C, and annealing at this temperature for 10-12 hours (Figure 2), allows it to react further with oxygen from the air, reducing d to less than 0.1. The overall reaction (with only the metal ions balanced) is given by:
0.5 Y2O3 + 2 BaO2 + 3 CuO ------> YBa2Cu3O7-d [1]
This compound is often called the 1-2-3 material, from the molar ratios of Y:Ba:Cu.
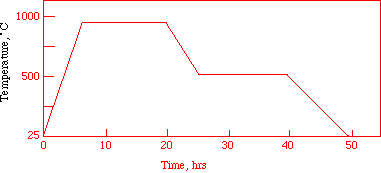
Figure 2. The heating-cooling sequence for the synthesis of the 1-2-3
superconductor involves several steps over two days.
The unit cell structure of YBa2Cu3O7 is based on the perovskite structure (Figure 3). Although it remains a controversial subject, the main feature of the structure believed to give rise to superconductivity is the existence of two-dimensional copper oxide sheets extending infinitely through the material in the ab plane. These sheets arise from the oxygen deficiency, as compared to the perovskite structure.
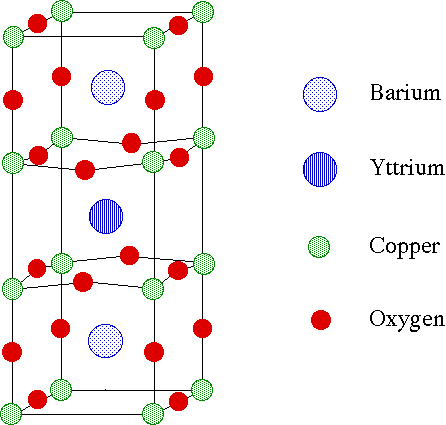
Figure 3. The unit cell of YBa2Cu3O7 is based on the ABX3 perovskite structure.
Experimental
Preparation of the superconductor
CAUTION!: Many chemicals are toxic. Avoid creating or breathing dust when grinding. Avoid eye and skin contact. Wash hands thoroughly after handling.
Weigh out onto a piece of weighing paper, 0.60 g of yttrium hydroxide, Y(OH)3, and transfer to a small beaker (be sure the beaker is dry). Add stoichiometrically equivalent amounts (1Y : 2Ba : 3Cu) of barium peroxide, BaO2, and cupric oxide, CuO, transferring each in turn to the beaker.
These three materials must now be thoroughly mixed to obtain good results. In a fumehood, place the powdered materials in a mortar. Mix and grind the material with a pestle for about 10 minutes; use a spatula to scrape the material off the sides of the mortar when it cakes. Your final powder should be a uniform color, with no lumps and no black or white spots or patches visible. What color is your powder, and why must you mix the starting powders?
You will now make 2 pellets with this powder. Scrape the powdered mixture onto a creased piece of weighing paper. Divide the mixture approximately in half using a second piece of weighing paper, one for each pellet. Press each mixture into a pellet using the pellet press. Transfer the finished pellets into a porcelain or alundum boat. NB: the pressed pellet is quite fragile and may shear crosswise or crumble when ejected from the die. If so, crush it in your mortar and repress. Heat the boat in a furnace to 150°C for 30 minutes to remove moisture. Heat to 930°C and hold for 12-16 hours (this time can be as long as 48 hours); then cool at a rate of 50°C/hour to 500°C, and hold for 12-16 hours. Finally, cool at a rate of 100°C/hour to room temperature. The cooled pellets, in their boats, will be stored in a desiccator until the next laboratory period. The finished pellet should be dark gray to black in color. A dark green material indicates a second, contaminant phase of composition Y2BaCuO5, which does not superconduct.
Discussion
Model Building A. The Perovskite Structure
The perovskite structure is named after the mineral CaTiO3. This structure is composed of corner-sharing TiO6 octahedra, with Ca ions in the large cavities at the corners of the unit cell (Figure 4).
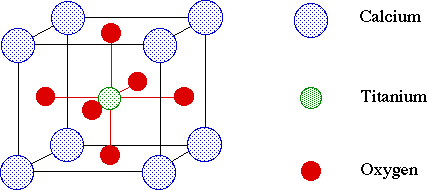
Figure 4. The perovskite structure is named after the mineral CaTiO3.
Questions:
1. Determine the oxidation state of Ti, if Ca and O have their normal oxidation states.
2. What is the basic unit cell structure for this type of compound?
3. What is the coordination number of calcium, titanium and oxygen.
4. Show that the unit cell of this compound corresponds to the formula CaTiO3. Remember that an ion shown as part of a unit cell does not contribute a whole ion to the cell unless it is wholly enclosed within the cell as the Ti ion is here. [Remember: How many unit cells is each atom in?].
5. How does the structure of WO3 differ from that of CaTiO3?
Model Building B. The Structure of the Superconductor YBa2Cu3O7
The 1-2-3 superconductor has a structure similar to perovskite. The resulting unit cell consists of three stacked cubic unit cells; it is considered to be orthorhombic rather than cubic, having an almost square base, but rectangular sides (a = 3.817Å, b = 3.882Å, c = 11.671Å).
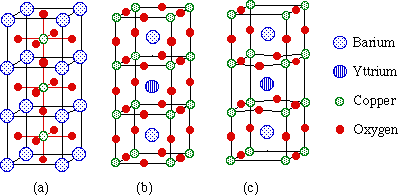
Questions:
1. The copper in YBa2Cu3O7 may be considered to be a mixture of +2 and +3 oxidation states. If Y has a +3 oxidation state and Ba and O have their normal oxidation states, what fraction of the copper is in each of the two oxidation states?
2. In your laboratory notebook, draw the idealized tetragonal unit cell if the yttrium and barium cubic structures were based exactly on the perovskite sub-structure.
3. Compare the coordination numbers of barium and yttrium in both the ideal perovskite sub-structure and the actual structure.
4. Comparing the two structures, speculate as to why the 1-2-3 oxide structure may exhibit superconductivity.
5. Show that the orthorhombic unit cell corresponds to the formula YBa2Cu3O7. Remember that an atom shown as part of a unit cell is shared equally between neighbouring unit cells, unless it is entriely enclosed within the unit cell.
Properties of a superconductor
CAUTION! Liquid nitrogen is extremely cold: 77 K = -196°C = -321°F. Do not spill it on your skin or clothing. Severe frostbite or freezing of the flesh can occur. Remove clothing that comes into contact with liquid nitrogen, as the liquid may be held within the spaces in the fabric, freezing the skin underneath. A drop or two spilled on the skin is not dangerous because the outer layer of the drop will vaporize, forming an insulating layer of gas.
A. The Meissner Effect
Using the plastic forceps, remove the superconductor pellet from the beaker and place it in the cut-off Styrofoam cup. If there is loose material on the pellet, scrape it off gently with a spatula. If the pellet has sheared, use the thickest piece and place it flat side up. Then place a small magnet on top of the pellet with the plastic forceps.
Have your teaching assistant pour some liquid nitrogen from the Dewar flask into a second Styrofoam cup. Carefully pour some of the liquid nitrogen into the cut-off cup, covering the pellet. Some of it will boil away as the pellet, magnet, and cup cool; add more as necessary, until the system is stable. When the pellet cools below the critical temperature, the magnet should “levitate” above the superconductor. Touch it gently with your forceps and it should spin. Remember to record all your observations, as you make them, in your laboratory notebook. Allow the liquid nitrogen to evaporate and the pellet and magnet to warm to room temperature before handling them with your fingers.
B. Loss of Electrical Resistance by the 4-probe Technique (demonstration by TA)
The loss of resistance below the critical temperature can be measured by measuring the voltage drop across the pellet in a circuit (Figure 6). A battery generates a current, I, which passes through the pellet. The voltage drop, V=IR, along the pellet due its resistance, R, at room temperature is measured with the voltmeter. When the material becomes superconducting, R=0, so V= 0, and no voltage drop should be measured. (The resistor in the circuit prevents the battery from being shorted when the superconductor loses its resistance. We use a 4-probe technique to eliminate contact resistances.)
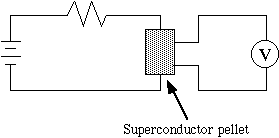
Figure 6. The 4-probe conductance test circuit is used to observe the loss of electrical resistance.
CAUTION! The resistor in the test circuit becomes quite hot. Do not touch the resistor or spill liquid nitrogen on it. Thermal shock could cause it to break, and you could burn your fingers.
The pellet is carefully inserted into the 4-pronged holder; the outer leads are connected to the battery and the inner leads to the voltmeter. Reverse the battery leads if the voltage is negative. After placing the holder in the Styrofoam cup, liquid nitrogen is slowly and carefully poured into the cup, as in the Meissner experiment. When the pellet cools below Tc, it should become superconducting; note what happens to the voltmeter reading at this point. The TA will then disassemble the experiment as follows: (1) disconnect the battery to prevent overheating the resistor; (2) let the nitrogen evaporate; (3) let the sample and holder return to room temperature; (4) remove the sample from the holder.
Some Questions to Incorporate into your Report:
1. Does your product exhibit the characteristics of superconductivity? Does it appear to be fully or only partially superconducting? If not, what do you hypothesize went wrong? How would you identify the presence of second phases?
2. Why did you compress your reactants into pellets for your solid state reaction?
3. A dream of materials chemists is to discover a material that would be superconducting above room temperature, so that no refrigerant would be required. Suggest how the properties of such superconductors could be valuable in each of the following fields: computers/electronics, power generation/transmission, and rail transportation.
4. Propose some reasonable syntheses that will lead to materials that could be a room temperature superconductor. Use the basic perovskite strucure and consider the following in your choice of metal ions: a) oxidation state; b) ratio of the cation/anion radius; c) ionization potential of the cation; d) the type of hole it is fitting into. Model the system using CAChe software, to see how your choice of metal ions has effected the crystal structure. For this structure, the space group is Pmmm, a=3.820, b=3.886, c=11.683. The fractional coordinates are as follows:
Atom x y z
________________________
Y 0.5
0.5 0.5
Ba 0.5 0.5 0.18393
Cu(1) 0 0 0
Cu(2) 0 0 0
O(1) 0 0.5 0
O(2) 0.5 0 0.37819
O(3) 0 0.5 0.37693
O(4) 0 0 0.15840
Further Information About Superconductors:
http://www.superconductors.org/index.htm#top