
| HOME | SITEMAP | CONTACT US | DIRECTIONS |
chemistry department homepage >> faculty >> Wayne Jones >> research
| Recent Research |
| Recent Teaching |
| Group Members |
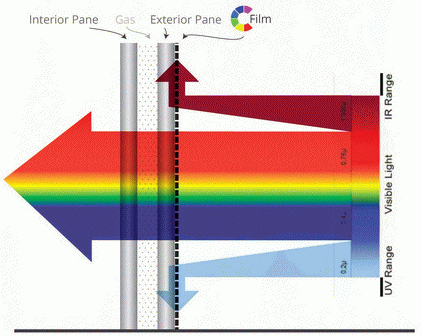
Light Absorbing Dye/Nano-materials
The current market for light absorbing dyes that maintain high photostability after thermal processing requires expensive dyes such as porphyrins complexed with precious metals. Organic dyes such as cyanine, phthalocyanine and aniline dyes can be produced at a lower cost but degrade readily at high temperatures, limiting their applications to films or low melt polymers. There are many applications that use ultraviolet, visible and near infrared absorbing dyes that filter light for applications such as laser protective shields, waveguide enhancement and energy control. These approaches to optical control allow for a multitude of potential products such as eye shields that protect from flash blindness, plasma and LCD pass filters, optical clothing and passive solar materials. By enhancing the thermal stability, these new markets can be assessed with cheaper, greener and more easily accessible light absorbing material.
The Wayne Jones research group has developed new materials from commercially available dyes which are stabilized through a reaction with metal oxide to form a novel nano-material: the focus is to develop and manufacture commercial quantities of ultraviolet, visible and near infrared absorbing dyes bound to metal oxide as a nano-material to filter or transform specific key wavelengths in optical devices. This process enhances the thermal stability of cyanine dyes from 100 °C up to 300 °C, allowing for incorporation of these stabilized dyes into inks, polymer pellets and films with protection from thermal degradation. This thermal stability allows the nano-material incorporation into high performance polymers such as acrylics, polycarbonates, polyimides and Teflon which would allow use of inexpensive dyes to take advantage of the enhanced polymer structural stability for versatile and durable applications.
Photocatalytic activity of TiO2 Nanofiber
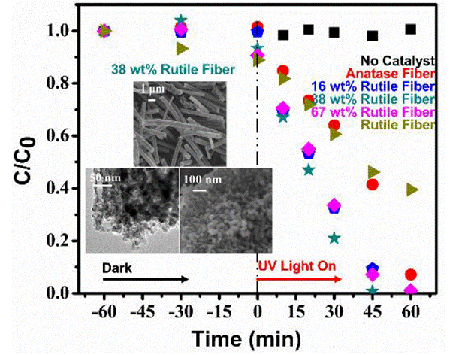
TiO2 polycrystalline sub-micron fibers can be used as photocatalysts for the degradation of a variety of organic molecules. Here we report on the optimization of these fibers for decontaminating pharmaceutical agents in aqueous medical waste streams. Mixed-phase TiO2 fibers have been prepared via a sol–gel technique followed by electrospinning and calcination. By adjusting the calcination temperature, the rutile phase fraction in TiO2 fibers can be tuned relative to the anatase phase from 0% to 100%. The effect of rutile phase fraction on grain size and specific surface area as well as their subsequent influences on the photocatalytic activity was investigated.
An optimal grain size in post-calcined TiO2 fibers was found to be critical to balance the e-/h+ volume recombination, surface recombination rate, and charge diffusion rate. The photocatalytic activities of the post-calcined TiO2 fibers with different rutile fractions were measured by monitoring the decreasing concentration of phenazopyridine in aqueous solution under UV illumination using UV–vis absorption spectroscopy. Post-calcined TiO2 fibers composing of 38 wt% rutile and 62 wt% anatase exhibited the highest initial degradation rate constant of 0.044 min-1. This optimal photocatalytic activity can be attributed to the combined influences of the fibers' phase composition, surface area and grain size.
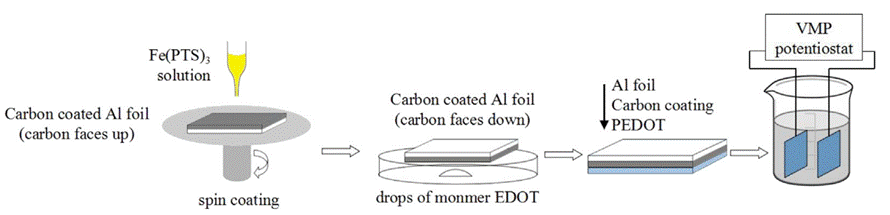
VPP PEDOT for Supercapacitors
Fast and efficient energy storage is an important part of our modern energy storage strategy. Supercapacitors are attracting more attention as key devices for energy storage due to their high power density and charge/discharge efficiency.
In this work, Laminar composite electrodes are prepared for application in supercapacitors using a catalyzed vapor-phase polymerization (VPP) of 3,4-ethylenedioxythiophene (EDOT) on the surface of commercial carbon coated aluminum foil. The processing temperature of VPP shows a significant effect on PEDOT morphology, the degree of orientation and its electrical properties. The relatively high temperature leads to high specific area and large conductive domains of PEDOT layer which benefits the capacitive behavior greatly according to the data presented. Since the substrate is already highly conductive, the PEDOT based composite can be used as electrode materials directly without adding current collector. By this simple and efficient process, PEDOT based composites exhibit specific capacitance up to 134 F g−1 with the polymerization temperature of 110 oC.
"Turn-on" Polymer Chemosensors
We are working on design and synthesis of conjugated polymers as fluorescence "turn-on" chemosensors as part of our sensor project. A fluorescence "turn-on" sensor is more desirable than a fluorescence quenching based "turn-off" sensor from the viewpoint of detection. There are many literature reports on fluorescent small molecule "turn-on" sensors based on photoinduced electron transfer (PET).
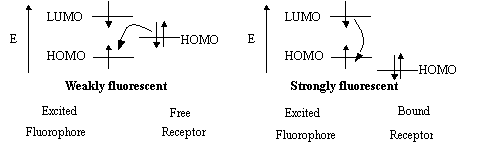
Our previous work and recent literature reports have been used to develop a strategy to synthesize a series of fluorescence "turn-on" polymer sensors with the PPETE polymer backbone as the fluorophore and different amino groups pendant to the backbone as receptors. For the initial study two new polymers, dea-PPETE and tmeda-PPETE, using N, N-diethylamino and N, N, N'-trimethylethylenediamino groups as receptors respectively, have been successfully synthesized and characterized. Photophysical studies show that the fluorescence of tmeda-PPETE was turned on upon binding several cations.
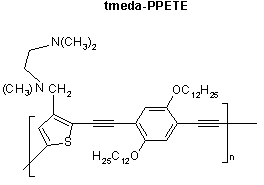
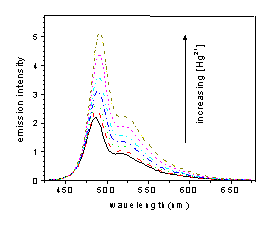
Future work is being directed to increase the sensitivity and selectivity of this class of chemosensors. Selectivity can be achieved by synthetically introdution different amino groups to the polymer system. The extent of fluorescence enhancement of the polymer depends on how efficiently the PET process is "switched off". This is a function of the lowering of HOMO energy levels upon binding cations. Given the different association constants between the cations and the amino receptors, the related coordination to different cations will also be investigated. We also reduced the percentage loading of receptors along the conjugated polymer backbone. Theoretically, the fluorescence of the polymer could be changed from a complete "on" state to a complete "off" state by adding a very small amount of cation, which cannot be achieved in small molecule sensors. Each of these new systems has been be characterized by NMR, elemental analysis and the fluorescence enhancement has been evaluated against Hg2+, Zn2+, H+, et al.
Dye Sensitized Solar Cells
Dye Sensitized Solar Cells (DSSC) use an inorganic dye, typically a ruthenium (II) polypyridine complex, which acts as an absorber to excite an electron from the HOMO to the LUMO. The other layers of the device help separate this pair for energy generation. Typically the layers used in a DSSC include an electron conducting layer (usually TiO2 or ZnO), a liquid hole conduction layer (usually I-/I3- ), a transparent electrode (usually indium tin oxide), and a metal counter electrode.
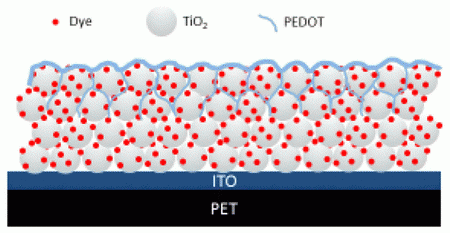
Vapor phased polymerized PEDOT as a hole conductor is incorporated within a bulk heterojunction comprised of the PEDOT, TiO2 porous electron conducting layer and ruthenium dyes. Here we will investigate PEDOTs ability to penetrate into the porous matrix of TiO2 to achieve a higher charge transfer rate in solid state-DSSC. We will determine the effect of the oxidants on the TiO2 layer and how this affects the overall efficiency. We will use cross section and scanning electron microscopy coupled with EDX to determine the polymerization extent, XPS to determine interactions between layers and the solar simulator to test the change in efficiencies as each layer is altered.

© Department of Chemistry, State University of New York at Binghamton, Binghamton, NY 13902-6000
Updated 04-12-2016